Short-term exposure to low doses of aflatoxin B1 aggravates nonalcoholic steatohepatitis by TLR4-mediated necroptosis
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Aflatoxin B1 (AFB1), a worldwide mycotoxin found in food and foodstuffs, is a potent hepatotoxin in humans and animals. Non-alcoholic fatty liver disease (NAFLD), a widespread disease, could progress from simple steatosis to non-alcoholic steatohepatitis (NASH), hepatic cirrhosis, and even hepatocellular carcinoma (HCC). To date, little is known concerning the relationship between AFB1 and the progression of NAFLD. The effects of low doses of AFB1 on the development of NASH and their mechanism were investigated in vivo and in vitro. The results in vivo showed that AFB1 at 20 and 40 μg/kg.bw aggravated CDAHFD-induced NASH in mice as demonstrated by increasing the serum and liver lipid accumulation, liver inflammation and injury. The results in vitro showed that AFB1 at 1.0 μM aggravated FFA-induced lipid accumulation, inflammation and cell damage in HepG2 cells. In addition, RNA-seq indicated that necroptosis, Toll like receptor signaling and TNF-α signaling showed a significant change in KEGG pathway enrichment in AFB1 at 40 μg/kg.bw. AFB1 significantly upregulated the mRNA and protein levels of TLR4, RIPK3, p-RIPK3, MLKL and p-MLKL, and increased TUNEL positive cells. Also, immunofluorescence results showed that TUNEL, TLR4, TNF-α had co-localized with RIPK3, respectively. Necroptosis inhibitor (GSK-872) attenuated the aggravating effects of AFB1 on NASH. Knockout of TLR4 inhibited necroptosis and rescued the aggravating effects of AFB1 on NASH. These data indicate that low dose of AFB1 aggravated NASH via TLR4-mediated necroptosis. This suggests that low dose of AFB1 is potentially harmful to animals and humans, as they exacerbate NASH.
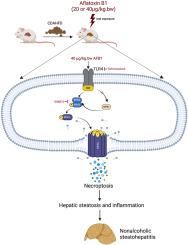
短期暴露于低剂量黄曲霉毒素 B1 会通过 TLR4 介导的坏死而加重非酒精性脂肪性肝炎。
黄曲霉毒素 B1(AFB1)是一种存在于食物和食品中的世界性霉菌毒素,对人类和动物都是一种强烈的肝脏毒素。非酒精性脂肪肝(NAFLD)是一种广泛存在的疾病,可从单纯的脂肪变性发展为非酒精性脂肪性肝炎(NASH)、肝硬化,甚至肝细胞癌(HCC)。迄今为止,人们对 AFB1 与非酒精性脂肪肝进展之间的关系知之甚少。研究人员在体内和体外研究了低剂量 AFB1 对非酒精性脂肪肝发展的影响及其机制。体内研究结果表明,20 和 40 μg/kg.bw 的 AFB1 会加重 CDAHFD 诱导的小鼠 NASH,表现为增加血清和肝脏脂质积累、肝脏炎症和损伤。体外研究结果表明,1.0 μM 的 AFB1 会加重 FFA 诱导的 HepG2 细胞脂质积累、炎症和细胞损伤。此外,RNA-seq表明,在40 μg/kg.bw的AFB1浓度下,坏死、Toll样受体信号转导和TNF-α信号转导的KEGG通路富集发生了显著变化。AFB1 能明显上调 TLR4、RIPK3、p-RIPK3、MLKL 和 p-MLKL 的 mRNA 和蛋白水平,并增加 TUNEL 阳性细胞。免疫荧光结果显示,TUNEL、TLR4、TNF-α分别与RIPK3共定位。坏死抑制剂(GSK-872)减轻了AFB1对NASH的加重作用。敲除 TLR4 可抑制坏死突变,并缓解 AFB1 对 NASH 的加重作用。这些数据表明,低剂量的AFB1通过TLR4介导的坏死细胞增多加重了NASH。这表明低剂量 AFB1 对动物和人类都有潜在危害,因为它们会加重 NASH。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


