A Universal Interfacial Reconstruction Strategy Based on Converting Residual Alkali for Sodium Layered Oxide Cathodes: Marvelous Air Stability, Reversible Anion Redox, and Practical Full Cell
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Mn-based layered oxide cathodes have attracted widespread attention due to high capacity and low cost, however, poor air stability, irreversible phase transitions, and slow kinetics inhibit their practical application. Here, we propose a universal interfacial reconstruction strategy based on converting residual alkali to tunnel phase Na0.44MnO2 for addressing the above mentioned issue simultaneously, using O3 NaNi0.4Fe0.2Mn0.4O2@2 mol % Na0.44MnO2 (NaNFM@NMO) as the prototype material. The optimized material exhibits an initial capacity and energy density comparable with lithium-ion batteries. The reversible anionic redox behavior and charge compensation mechanism of NaNFM@NMO were analyzed and verified by soft X-ray absorption spectrum and in situ X-ray absorption spectrum. Due to the intrinsic stability of the tunnel structure, excellent air stability and highly reversible structure evolution of the NaNFM@NMO cathode material are achieved, which are confirmed by contact angle test, rigorous aging test, and in situ X-ray diffraction. More importantly, the NaNFM@NMO cathode demonstrates a great match with the nonpresodiated hard carbon anode and shows excellent electrochemical performance of the full cell. Additionally, such a strategy could be also applied to modify P2-type cathodes, showing superior universality and good prospects in industrialized production. Overall, the proposed strategy could improve air stability while remaining interfacial and bulk stable simultaneously and will open up a whole new field for the optimization of other electrode materials.
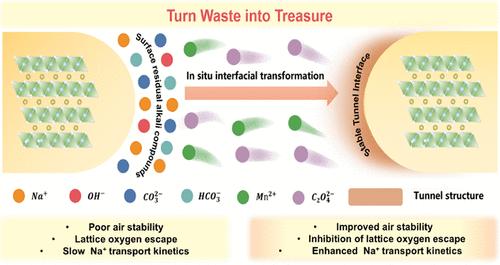
基于钠层状氧化物阴极残碱转化的通用界面重构策略:神奇的空气稳定性、可逆的阴离子氧化还原和实用的全电池
锰基层状氧化物阴极因其高容量和低成本而受到广泛关注,但其空气稳定性差、不可逆相变和缓慢的动力学特性阻碍了其实际应用。在此,我们以 O3 NaNi0.4Fe0.2Mn0.4O2@2 mol % Na0.44MnO2(NaNFM@NMO)为原型材料,提出了一种基于将残碱转化为隧道相 Na0.44MnO2 的通用界面重构策略,以同时解决上述问题。优化后的材料显示出与锂离子电池相当的初始容量和能量密度。软 X 射线吸收光谱和原位 X 射线吸收光谱分析并验证了 NaNFM@NMO 的可逆阴离子氧化还原行为和电荷补偿机制。由于隧道结构的内在稳定性,NaNFM@NMO 阴极材料实现了优异的空气稳定性和高度可逆的结构演化,这一点通过接触角测试、严格的老化测试和原位 X 射线衍射得到了证实。更重要的是,NaNFM@NMO 阴极与非阳极化硬碳阳极非常匹配,在整个电池中显示出优异的电化学性能。此外,这种策略还可用于改性 P2 型阴极,显示出优越的通用性和良好的工业化生产前景。总之,所提出的策略可以在同时保持界面和块体稳定的情况下提高空气稳定性,并将为其他电极材料的优化开辟一个全新的领域。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


