The definition of chemical contaminants in food: Ambiguity and consequences
IF 3
4区 医学
Q1 MEDICINE, LEGAL
引用次数: 0
Abstract
Consumers may be exposed via foods to a diverse range of substances that could be considered as contaminants. However, it is not always straightforward to understand the definition of a ‘contaminant’. The present review evaluates how various categories of food-relevant substances are considered in terms of being ‘contaminants’. To this end these categories of food borne constituents are evaluated against the various criteria encountered in the available definitions of a food contaminant, including unintentional presence, harmful, existence of regulatory limits, and stakeholder perception. The categories of chemicals considered include: phytotoxins, mycotoxins, (heavy) metals, persistent organic pollutants (POPs), processing aids, process related contaminants, food contact materials (FCMs), pesticides and veterinary drugs. The evaluation revealed that usage of the term appears complex, and may differ between stakeholders. A common proposed definition of the term ‘contaminant’ could be ‘a substance considered to require control measures due to the unacceptability of its context within a food’. Use of a dimension of harm results in equivocal outcomes because risk depends on the level of exposure. As the term ‘contaminant’ has influence on risk management including public policy, the motivations for applying the term should be subject to more detailed analysis and understanding.
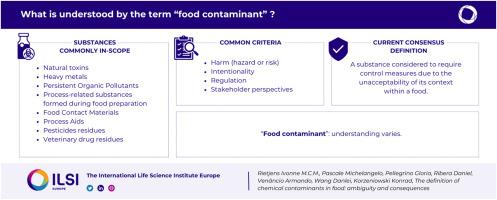
食品中化学污染物的定义:模糊性和后果。
消费者可能会通过食品接触到各种可被视为污染物的物质。然而,要理解 "污染物 "的定义并非总是那么简单。本综述从 "污染物 "的角度评估了各类食品相关物质。为此,我们根据现有食品污染物定义中的各种标准,包括无意存在、有害、存在监管限制和利益相关者的看法,对这些类别的食源性成分进行了评估。考虑的化学品类别包括:植物毒素、霉菌毒素、(重)金属、持久性有机污染物、加工助剂、加工相关污染物、食品接触材料、杀虫剂和兽药。评估显示,该术语的用法似乎很复杂,而且利益相关者之间可能存在差异。污染物 "一词的常见拟议定义可以是 "由于其在食品中的不可接受性而被认为需要采取控制措施的物质"。使用危害维度会导致结果不明确,因为风险取决于暴露程度。由于 "污染物 "一词会对包括公共政策在内的风险管理产生影响,因此应更详细地分析和理解使用该词的动机。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.70
自引率
8.80%
发文量
147
审稿时长
58 days
期刊介绍:
Regulatory Toxicology and Pharmacology publishes peer reviewed articles that involve the generation, evaluation, and interpretation of experimental animal and human data that are of direct importance and relevance for regulatory authorities with respect to toxicological and pharmacological regulations in society. All peer-reviewed articles that are published should be devoted to improve the protection of human health and environment. Reviews and discussions are welcomed that address legal and/or regulatory decisions with respect to risk assessment and management of toxicological and pharmacological compounds on a scientific basis. It addresses an international readership of scientists, risk assessors and managers, and other professionals active in the field of human and environmental health.
Types of peer-reviewed articles published:
-Original research articles of relevance for regulatory aspects covering aspects including, but not limited to:
1.Factors influencing human sensitivity
2.Exposure science related to risk assessment
3.Alternative toxicological test methods
4.Frameworks for evaluation and integration of data in regulatory evaluations
5.Harmonization across regulatory agencies
6.Read-across methods and evaluations
-Contemporary Reviews on policy related Research issues
-Letters to the Editor
-Guest Editorials (by Invitation)

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


