An integrated proteomic and phosphoproteomic landscape of chronic kidney disease
IF 2.8
2区 生物学
Q2 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
Abstract
The prevalence of chronic kidney disease (CKD) is gradually rising worldwide. Patients often remain asymptomatic for an extended period, leaving them unaware of their condition, which can lead to progressing to end-stage renal disease and cause significant economic burden. Improved understanding of CKD pathogenesis can enhance early detection and facilitate advances in drug development. Here, we performed proteomic and phosphoproteomic analyses of the mouse unilateral ureteral obstruction model to explore the molecular mechanisms of chronic kidney injury. 474 significantly differentially expressed proteins and 96 significantly differentially expressed phosphoproteins were screened, respectively. Chronic kidney injury involves complex metabolic pathways such as citrate cycle and hematopoietic system in proteome, and mitochondrial oxidative phosphorylation suppression is a notable alteration. The phosphoproteomic analysis revealed a significant upregulation in epithelial mesenchymal transition and P53 pathways, with a corresponding increase in the phosphorylation of Jun at serine 73. Utilizing HK2 cells, we observed that the reduction oxidative phosphorylation was consistently associated with an augmentation in oxidative stress, which subsequently activated Jun and induced apoptosis. Proteins that act as hubs in these pathways may be candidate targets for CKD intervention. These findings contribute significantly to the current understanding of CKD and provide valuable insights for future studies.
Significance
Chronic kidney disease (CKD) incidence rising annually with varied etiologies, kidney often irreversibly fibrotic, the treatment options are limited and often ineffective due to deficient understanding of renal fibrosis mechanisms. Despite the extensive efforts and numerous omics studies conducted on renal fibrosis, to date, no study has been undertaken to investigate the role of phosphorylated proteins in UUO models. Previously, we performed a comprehensive transcriptome and proteome analysis based on the CKD model, but the potential alterations in the phosphoproteome were not addressed. Here, an integrated proteomic and phosphoproteomic landscape of CKD was completed, which was the the first phosphoproteomic profiles of UUO model. Phosphoproteomic profile suggests that the epithelial mesenchymal transition and P53 pathways is significantly activated in mouse models of kidney injury, and the core protein Jun played a key role in CKD. And a preliminary correlation between P-Jun and oxidative phosphorylation was found base on HK2 cells. Our work contributes to a deeper understanding of the disease characteristics and molecular mechanisms of CKD. Identifying potential CKD targets from proteome and phosphoproteome may provide valuable insights for early diagnosis and treatment of CKD.
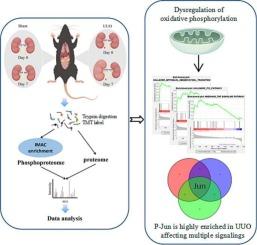
慢性肾脏病的综合蛋白质组和磷酸蛋白质组图谱。
慢性肾脏病(CKD)的发病率在全球范围内逐渐上升。患者往往长期无症状,对自己的病情毫无察觉,这可能导致病情恶化为终末期肾病,并造成巨大的经济负担。加深对慢性肾功能衰竭发病机制的了解可以提高早期发现率,促进药物开发。在此,我们对小鼠单侧输尿管梗阻模型进行了蛋白质组学和磷酸化蛋白质组学分析,以探索慢性肾损伤的分子机制。分别筛选出了 474 个明显差异表达的蛋白质和 96 个明显差异表达的磷酸蛋白。慢性肾损伤在蛋白质组中涉及复杂的代谢途径,如柠檬酸循环和造血系统,线粒体氧化磷酸化抑制是一个明显的改变。磷酸化蛋白质组分析显示,上皮间质转化和 P53 通路显著上调,Jun 在丝氨酸 73 处的磷酸化也相应增加。利用 HK2 细胞,我们观察到氧化磷酸化的减少始终与氧化应激的增加有关,氧化应激随后激活 Jun 并诱导细胞凋亡。在这些通路中起枢纽作用的蛋白质可能是干预 CKD 的候选靶标。这些发现极大地促进了目前对 CKD 的了解,并为今后的研究提供了宝贵的见解。意义:慢性肾脏病(CKD)的发病率每年都在上升,其病因多种多样,肾脏经常发生不可逆的纤维化,由于对肾脏纤维化机制的认识不足,治疗方案有限且往往无效。尽管对肾脏纤维化进行了大量的努力和omics研究,但迄今为止,还没有研究调查磷酸化蛋白在UUO模型中的作用。此前,我们基于 CKD 模型进行了全面的转录组和蛋白质组分析,但并未涉及磷酸化蛋白质组的潜在改变。在此,我们完成了 CKD 的综合蛋白质组和磷酸蛋白组图谱,这是 UUO 模型的首个磷酸蛋白组图谱。磷酸蛋白组图谱表明,上皮间充质转化和P53通路在小鼠肾损伤模型中被显著激活,核心蛋白Jun在CKD中起着关键作用。在HK2细胞的基础上,还初步发现了P-Jun与氧化磷酸化之间的相关性。我们的工作有助于加深对 CKD 疾病特征和分子机制的理解。从蛋白质组和磷酸化蛋白质组中识别潜在的 CKD 靶点可能为 CKD 的早期诊断和治疗提供有价值的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of proteomics
生物-生化研究方法
CiteScore
7.10
自引率
3.00%
发文量
227
审稿时长
73 days
期刊介绍:
Journal of Proteomics is aimed at protein scientists and analytical chemists in the field of proteomics, biomarker discovery, protein analytics, plant proteomics, microbial and animal proteomics, human studies, tissue imaging by mass spectrometry, non-conventional and non-model organism proteomics, and protein bioinformatics. The journal welcomes papers in new and upcoming areas such as metabolomics, genomics, systems biology, toxicogenomics, pharmacoproteomics.
Journal of Proteomics unifies both fundamental scientists and clinicians, and includes translational research. Suggestions for reviews, webinars and thematic issues are welcome.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


