Transient cerebral ischaemia alters mesenteric arteries in hypertensive rats: Limited reversal despite suberoylanilide hydroxamic acid cerebroprotection
IF 5.2
2区 医学
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
Abstract
Stroke induces brain injury, especially severe in hypertensive patients, and elevates mortality rates through non-neurological complications. However, the potential effects of a transient ischaemic episode on the peripheral vasculature of hypertensive individuals remain unclear. We investigated whether transient cerebral ischaemia (90 min)/reperfusion (1 or 8 days) induces alterations in mesenteric resistance artery (MRA) properties in adult male spontaneously hypertensive rats (SHR). In addition, we assessed whether the reported cerebroprotective effects of suberoylanilide hydroxamic acid (SAHA; 50 mg/kg; administered intraperitoneally at 1, 4, or 6 h after reperfusion onset) extend over several days and include beneficial effects on MRAs. Functional and structural properties of MRAs were examined at 1- and 8-days post-stroke. Nuclei distribution, collagen content, and oxidative stress were assessed. Ischaemic brain damage was evaluated longitudinally using magnetic resonance imaging. Following stroke, MRAs from SHR exhibited non-reversible impaired contractile responses to the thromboxane A2 receptor agonist U46619. Stroke increased the MRA cross-sectional area, wall thickness, and wall/lm ratio due to augmented collagen deposition. These changes were partially sustained 8 days later. SAHA did not improve U46619-induced contractions but mitigated stroke-induced oxidative stress and collagen deposition, preventing MRA remodelling at 24 h of reperfusion. Furthermore, SAHA induced sustained cerebroprotective effects over 8 days, including reduced brain infarct and oedema, and improved neurological scores. However, SAHA had minimal impact on chronic MRA contractile impairments and remodelling. These findings suggest that stroke causes MRA changes in hypertensive subjects. While SAHA treatment offers sustained protection against brain damage, it cannot fully restore MRA alterations.
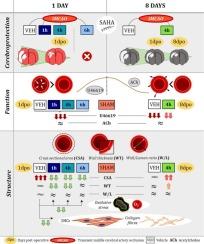
短暂性脑缺血改变了高血压大鼠的肠系膜动脉:尽管亚伯酰苯胺羟肟酸具有脑保护作用,但其逆转作用有限。
中风会导致脑损伤,对高血压患者的影响尤为严重,并通过非神经系统并发症提高死亡率。然而,一过性脑缺血对高血压患者外周血管的潜在影响仍不清楚。我们研究了一过性脑缺血(90 分钟)/再灌注(1 天或 8 天)是否会诱发成年雄性自发性高血压大鼠肠系膜阻力动脉(MRA)特性的改变。此外,我们还评估了已报道的亚伯酰苯胺羟肟酸(SAHA;50 毫克/千克;在再灌注开始后 1、4 或 6 小时腹腔给药)的脑保护作用是否会持续数天并包括对 MRA 的有益作用。卒中后 1 天和 8 天,对 MRA 的功能和结构特性进行了检测。评估了核分布、胶原含量和氧化应激。利用磁共振成像对缺血性脑损伤进行了纵向评估。中风后,SHR 的 MRA 对血栓素 A2 受体激动剂 U46619 表现出不可逆的收缩反应受损。由于胶原沉积增加,中风增加了 MRA 的横截面积、壁厚和壁/lm 比值。这些变化在 8 天后部分持续。SAHA 并未改善 U46619 诱导的收缩,但减轻了中风诱发的氧化应激和胶原沉积,防止了再灌注 24 小时后的 MRA 重塑。此外,SAHA 还能在 8 天内产生持续的脑保护作用,包括减少脑梗塞和水肿,改善神经系统评分。然而,SAHA 对慢性 MRA 收缩损伤和重塑的影响微乎其微。这些研究结果表明,中风会导致高血压患者的 MRA 发生变化。虽然 SAHA 治疗能持续保护患者免受脑损伤,但却不能完全恢复 MRA 的改变。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Life sciences
医学-药学
CiteScore
12.20
自引率
1.60%
发文量
841
审稿时长
6 months
期刊介绍:
Life Sciences is an international journal publishing articles that emphasize the molecular, cellular, and functional basis of therapy. The journal emphasizes the understanding of mechanism that is relevant to all aspects of human disease and translation to patients. All articles are rigorously reviewed.
The Journal favors publication of full-length papers where modern scientific technologies are used to explain molecular, cellular and physiological mechanisms. Articles that merely report observations are rarely accepted. Recommendations from the Declaration of Helsinki or NIH guidelines for care and use of laboratory animals must be adhered to. Articles should be written at a level accessible to readers who are non-specialists in the topic of the article themselves, but who are interested in the research. The Journal welcomes reviews on topics of wide interest to investigators in the life sciences. We particularly encourage submission of brief, focused reviews containing high-quality artwork and require the use of mechanistic summary diagrams.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


