Supramolecular arrangements in human amyloid tissues using SAXS
IF 2.2
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Amyloid diseases are characterized by the accumulation of misfolded protein aggregates in human tissues, pose significant challenges for both diagnosis and treatment. Protein aggregations known as amyloids are linked to several neurodegenerative conditions including Alzheimer's disease, Parkinson's disease, and systemic amyloidosis. The key goal of this research is to employ Small-Angle X-ray Scattering (SAXS) to examine the supramolecular structures of amyloid aggregates in human tissues. We present the structural analysis of amyloid using SAXS, which is employed directly to analyze thin tissue samples without damaging the tissues. This technique provides size and shape information of fibrils, which can be used to generate low-resolution 2D models. The present study investigates the structural changes in amyloid fibril axial d-spacing and scattering intensity in different human tissues, including kidney, heart, thyroid, and others, while also accounting for the presence of triglycerides in these tissues. Tissue structural components were examined at momentum transfer values between q = 0.2 nm−1 and 1.5 nm−1. The d-spacing is a critical parameter in SAXS that provides information about the periodic distances between structures within a sample. From the supramolecular SAXS patterns, the axial d-spacing of fibrils in amyloid tissues is prominent and exists within the 3rd to 10th order, compared to that of healthy tissues which do not have notable peak orders. The axial period of fibrils in amyloid tissues is within the scattering vector range 57.40–64.64 nm−1 while in normal tissues the range is between 60.68 and 61.41 nm−1, which is 3.0 nm−1 smaller than amyloid-containing tissues. Differences in d-spacing are often correlate with distinct pathological mechanisms or stages of disease progression. The application of SAXS to investigate amyloid structures in human tissues has enormous potential to further knowledge of amyloid disorders. This work will open the path for novel diagnostic instruments and therapeutic strategies meant to reduce the burden of amyloid-related diseases by offering a thorough structural examination of amyloid aggregates.
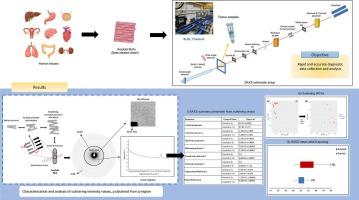
利用 SAXS 分析人体淀粉样组织中的超分子排列。
淀粉样蛋白疾病的特征是折叠错误的蛋白质聚集在人体组织中,给诊断和治疗带来了巨大挑战。被称为淀粉样蛋白的蛋白质聚集与多种神经退行性疾病有关,包括阿尔茨海默病、帕金森病和全身性淀粉样变性。这项研究的主要目标是利用小角 X 射线散射(SAXS)来研究人体组织中淀粉样蛋白聚集体的超分子结构。我们介绍了利用 SAXS 对淀粉样蛋白进行结构分析的方法,该方法可直接用于分析薄组织样本,而无需破坏组织。该技术提供了纤维的尺寸和形状信息,可用于生成低分辨率的二维模型。本研究调查了不同人体组织(包括肾脏、心脏、甲状腺等)中淀粉样蛋白纤维轴向 d-间距和散射强度的结构变化,同时还考虑了这些组织中甘油三酯的存在。在 q = 0.2 nm-1 和 1.5 nm-1 之间的动量传递值下对组织结构成分进行了研究。d 间距是 SAXS 中的一个关键参数,它提供了样品内结构间周期性距离的信息。从超分子 SAXS 图谱来看,淀粉样变组织中纤维的轴向 d-间距很明显,在 3 至 10 阶范围内,而健康组织中的纤维则没有明显的峰值阶数。淀粉样组织中纤维的轴向周期在散射矢量范围 57.40-64.64 nm-1 之间,而正常组织的范围在 60.68-61.41 nm-1 之间,比含淀粉样组织小 3.0 nm-1。d-间距的差异通常与不同的病理机制或疾病进展阶段相关。应用 SAXS 研究人体组织中的淀粉样蛋白结构对于进一步了解淀粉样蛋白疾病具有巨大的潜力。这项工作将为新型诊断仪器和治疗策略开辟道路,通过对淀粉样蛋白聚集体进行彻底的结构检查,减轻淀粉样蛋白相关疾病的负担。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biophysical chemistry
生物-生化与分子生物学
CiteScore
6.10
自引率
10.50%
发文量
121
审稿时长
20 days
期刊介绍:
Biophysical Chemistry publishes original work and reviews in the areas of chemistry and physics directly impacting biological phenomena. Quantitative analysis of the properties of biological macromolecules, biologically active molecules, macromolecular assemblies and cell components in terms of kinetics, thermodynamics, spatio-temporal organization, NMR and X-ray structural biology, as well as single-molecule detection represent a major focus of the journal. Theoretical and computational treatments of biomacromolecular systems, macromolecular interactions, regulatory control and systems biology are also of interest to the journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


