C–OH Bond Activation for Stereoselective Radical C-Glycosylation of Native Saccharides
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Radical C-glycosylation presents a flexible and efficient method for synthesizing C-glycosides. Existing methods always require multistep processes for generating anomeric radicals. In this study, we introduce a streamlined approach to produce anomeric radicals through direct C–OH bond homolysis of unmodified saccharides, eliminating the need for protection, deprotection, or activation steps. These anomeric radicals selectively couple with activated alkenes, yielding C-glycosylation products with high stereoselectivity (>20:1). This method is applicable to a variety of native monosaccharides, such as l-arabinose, d-arabinose, d-xylose, l-xylose, d-galactose, β-d-glucose, α-d-glucose, and l-ribose, as well as oligosaccharides including α-lactose, d-(+)-melibiose, and acarbose. We also extend this approach to C-glycosylation of amino acid and peptide derivatives, and demonstrate a streamlined synthesis of an anti-inflammatory agent.
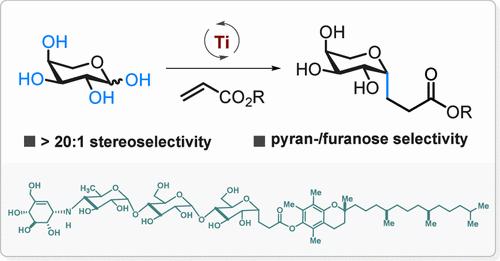
C-OH 键活化用于原生糖的立体选择性 Radical C-Glycosylation
自由基 C-糖苷化是合成 C-糖苷的一种灵活高效的方法。现有的方法总是需要经过多个步骤才能生成同分异构基。在本研究中,我们介绍了一种简化的方法,通过直接对未修饰的糖类进行 C-OH 键均裂来产生同分异构自由基,省去了保护、去保护或活化步骤。这些异构体自由基可选择性地与活化的烯烃偶联,产生具有高度立体选择性(20:1)的 C-糖基化产物。这种方法适用于多种原生单糖,如 l-阿拉伯糖、d-阿拉伯糖、d-木糖、l-木糖、d-半乳糖、β-d-葡萄糖、α-d-葡萄糖和 l-核糖,以及低聚糖,包括 α-乳糖、d-(+)-麦芽糖和阿卡波糖。我们还将这种方法扩展到氨基酸和肽衍生物的 C-糖基化,并展示了一种抗炎剂的简化合成方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


