Iridium-catalyzed highly enantioselective and chemodivergent coupling reaction of vinyl azides and vinyl benzoxazinones†
IF 4.7
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The first iridium-catalyzed enantioselective coupling reaction of vinyl azides and vinyl benzoxazinones is presented. Vinyl azides underwent a tandem allylic alkylation/interrupted Schmidt rearrangement process to produce enantioenriched 3,4-dihydroquinolin-2(1H)-imines, a new class of N-heterocycles. In the presence of CH3CO2H, a conventional asymmetric allylic substitution occurred to provide access to nonracemic allylic amides. The synthetic transformations of the product were carried out to construct chiral amines, amides and N-hetero polycycles.
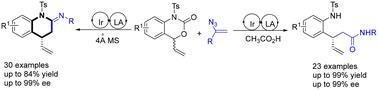
铱催化的乙烯基叠氮化物和乙烯基苯并噁嗪酮的高对映选择性和化学发散性偶联反应
本文首次介绍了铱催化的乙烯基叠氮化物和乙烯基苯并恶嗪酮的对映选择性偶联反应。乙烯基叠氮化物经过串联烯丙基烷基化/间歇施密特重排过程,生成了对映体富集的 3,4-二氢喹啉-2(1H)-亚胺,这是一类新的 N-杂环化合物。在 CH3CO2H 的存在下,发生了传统的不对称烯丙基取代反应,从而获得了非外消旋烯丙基酰胺。通过对产物进行合成转化,构建了手性胺、酰胺和 N-杂环。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Chemistry Frontiers
CHEMISTRY, ORGANIC-
CiteScore
7.90
自引率
11.10%
发文量
686
审稿时长
1 months
期刊介绍:
Organic Chemistry Frontiers is an esteemed journal that publishes high-quality research across the field of organic chemistry. It places a significant emphasis on studies that contribute substantially to the field by introducing new or significantly improved protocols and methodologies. The journal covers a wide array of topics which include, but are not limited to, organic synthesis, the development of synthetic methodologies, catalysis, natural products, functional organic materials, supramolecular and macromolecular chemistry, as well as physical and computational organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


