Suppression of intestinal Ticam1 ameliorated MASH via Akkermansia muciniphila QAA37749.1 mediated betaine transformation
IF 4.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular basis of disease
Pub Date : 2024-11-12
DOI:10.1016/j.bbadis.2024.167571
引用次数: 0
Abstract
Background & aims
Gut inflammation caused by diets could damage the intestinal barrier, which increases the liver exposition to pathogenic substances. Toll-IL-1 receptor (TIR) domain-containing adaptor molecule-1 (Ticam1) is a key molecule in the Toll-like receptors (TLRs) pathway, which is important for the immune defense against pathogens such as bacteria or viruses. In this study, mouse intestinal epithelial cell (IEC) Ticam1 was knocked out to suppress the intestinal inflammation response in metabolic dysfunction-associated steatohepatitis (MASH) to investigate its influence on the development of MASH.
Methods
The IEC-specific Ticam1 knockout (Ticam1ΔIEC) mice and the control (Ticam1fl/fl) mice were fed with high-fat high-fructose diet (HFD) for 22 weeks to evaluate the gut alteration and the MASH-associated disorders. The intestinal secreted immunoglobulin A (sIgA) and IgA-secreting immune cells were detected. Shotgun metagenomic sequencing was used to find the gut microbiome shift in different groups. Liquid chromatography mass spectrometry was also performed to evaluate the change of serum metabolites caused by the gut microbiome alteration.
Results
The gut inflammation and gut barrier dysfunction were both alleviated in HFD-fed Ticam1ΔIEC mice, which had improved MASH disorders compared with Ticam1fl/fl. Additionally, HFD-fed Ticam1ΔIEC mice had increased sIgA and intestinal IgA-secreting immune cells. It showed a significantly higher content of Akkermansia muciniphila. We proved that Akkermansia muciniphila encoded a protein named QAA37749.1 that could promote the conversion of choline to betaine, through which the development of MASH was inhibited in HFD-Ticam1ΔIEC mice.
Conclusion
Deletion of IEC Ticam1 alleviated MASH disorder and gut dysfunction in mice. It enhanced the level of intestinal sIgA and the growth of Akkermansia muciniphila, which supported the betaine transformation by QAA37749.1. Suppressing IEC Ticam1 might be a promising strategy for MASH disorder.
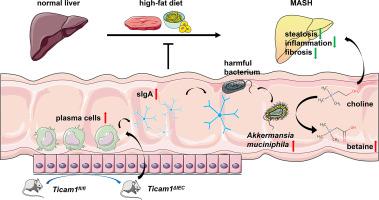
通过 Akkermansia muciniphila QAA37749.1 介导的甜菜碱转化,抑制肠道 Ticam1 可改善 MASH。
背景和目的:饮食引起的肠道炎症会破坏肠道屏障,从而增加肝脏对致病物质的暴露。含 Toll-IL-1 受体(TIR)结构域的适配分子-1(Ticam1)是 Toll 样受体(TLRs)通路中的一个关键分子,对抵御细菌或病毒等病原体的免疫防御非常重要。本研究通过敲除小鼠肠上皮细胞(IEC)Ticam1来抑制代谢功能障碍相关性脂肪性肝炎(MASH)的肠道炎症反应,以探讨其对MASH发病的影响:方法:用高脂高果糖饮食(HFD)喂养IEC特异性Ticam1基因敲除(Ticam1ΔIEC)小鼠和对照组(Ticam1fl/fl)小鼠22周,以评估肠道改变和MASH相关疾病。检测了肠道分泌的免疫球蛋白A(sIgA)和分泌IgA的免疫细胞。采用霰弹枪元基因组测序来发现不同组别中肠道微生物组的变化。此外,还进行了液相色谱质谱分析,以评估肠道微生物组改变引起的血清代谢物变化:结果:与Ticam1fl/fl小鼠相比,HFD喂养的Ticam1ΔIEC小鼠的肠道炎症和肠道屏障功能障碍都得到了缓解,MASH紊乱也得到了改善。此外,HFD饲喂的Ticam1ΔIEC小鼠的sIgA和肠道分泌IgA的免疫细胞增加。小鼠体内 Akkermansia muciniphila 的含量明显增加。我们证实,Akkermansia muciniphila编码一种名为QAA37749.1的蛋白质,该蛋白质可促进胆碱向甜菜碱的转化,通过这种转化,HFD-Ticam1ΔIEC小鼠MASH的发生受到抑制:结论:删除 IEC Ticam1 可减轻小鼠的 MASH 症状和肠道功能障碍。结论:删除 IEC Ticam1 可缓解小鼠的 MASH 症状和肠道功能障碍,提高肠道 sIgA 水平和 Akkermansia muciniphila 的生长,从而支持 QAA37749.1 的甜菜碱转化。抑制 IEC Ticam1 可能是一种治疗 MASH 疾病的有效策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
12.30
自引率
0.00%
发文量
218
审稿时长
32 days
期刊介绍:
BBA Molecular Basis of Disease addresses the biochemistry and molecular genetics of disease processes and models of human disease. This journal covers aspects of aging, cancer, metabolic-, neurological-, and immunological-based disease. Manuscripts focused on using animal models to elucidate biochemical and mechanistic insight in each of these conditions, are particularly encouraged. Manuscripts should emphasize the underlying mechanisms of disease pathways and provide novel contributions to the understanding and/or treatment of these disorders. Highly descriptive and method development submissions may be declined without full review. The submission of uninvited reviews to BBA - Molecular Basis of Disease is strongly discouraged, and any such uninvited review should be accompanied by a coverletter outlining the compelling reasons why the review should be considered.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


