GPR68 supports AML cells through the calcium/calcineurin pro-survival pathway and confers chemoresistance by mediating glucose metabolic symbiosis
IF 4.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular basis of disease
Pub Date : 2024-11-08
DOI:10.1016/j.bbadis.2024.167565
引用次数: 0
Abstract
Accumulating evidence demonstrates that the “Warburg effect” that glycolysis is enhanced even in the presence of oxygen existed in hematopoietic malignancies, contributing to extracellular acidosis. G-protein coupled receptor 68 (GPR68), as a proton sensing GPCR responding to extracellular acidosis, is expected to play a critical role in hematopoietic malignancies. In the present study, we found that GPR68 was overexpressed in acute myeloid leukemia (AML) cells, and GPR68 deficiency impaired AML cell survival in vitro and cell engraftment in vivo. Mechanistic studies revealed that unlike GPR68 regulates Calpain1 in myelodysplastic syndromes (MDS) cells, GPR68 deficiency reduced cytosolic Ca2+ levels and calcineurin (CaN) activity in AML cells through an NFAT-independent mechanism. Moreover, the decreased Ca2+ levels disturbed cellular respiration (i.e., oxidative phosphorylation, OxPhos) by inhibiting isocitrate dehydrogenase (IDH) activity; this was more pronounced when BCL2 was inhibited simultaneously. Interestingly, GPR68 inhibition also decreased aerobic glycolysis in AML cells in a Ca2+-independent manner, suggesting that GPR68 mediated glucose metabolic symbiosis. As glucose metabolic symbiosis and the heterogeneous dependencies on aerobic glycolysis and cellular respiration tremendously impact chemosensitivity, the inhibition of GPR68 potentiated the tumoricidal effect of first-line chemotherapeutic agents, including BCL-2 inhibitors targeting OxPhos and cytarabine (Ara-C) targeting glycolysis. Consistent with these in vitro observations, higher levels of GPR68 were associated with inferior clinical outcomes in AML patients who received chemotherapies. In short, GPR68 drives the Ca2+/CaN pro-survival pathway and mediates glucose metabolic pathways in AML cells. Targeting GPR68 eradicates AML cells and alleviates chemoresistance, which could be exploited as a therapeutic target.
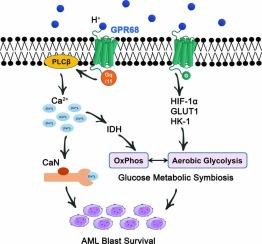
GPR68 通过钙/钙神经蛋白促生存途径支持急性髓细胞白血病细胞,并通过介导葡萄糖代谢共生赋予化疗抗性。
越来越多的证据表明,造血恶性肿瘤中存在 "沃伯格效应",即即使在有氧的情况下,糖酵解也会增强,从而导致细胞外酸中毒。G 蛋白偶联受体 68(GPR68)作为一种质子感应 GPCR,可对细胞外酸中毒做出反应,预计将在造血恶性肿瘤中发挥关键作用。在本研究中,我们发现 GPR68 在急性髓性白血病(AML)细胞中过表达,GPR68 缺乏会损害 AML 细胞的体外存活和体内细胞移植。机理研究发现,与 GPR68 在骨髓增生异常综合征(MDS)细胞中调控 Calpain1 不同,GPR68 缺乏会通过 NFAT 依赖性机制降低 AML 细胞的细胞膜 Ca2+ 水平和钙神经蛋白(CaN)活性。此外,Ca2+水平的降低通过抑制异柠檬酸脱氢酶(IDH)的活性干扰了细胞呼吸(即氧化磷酸化,OxPhos);当同时抑制 BCL2 时,这种情况更为明显。有趣的是,抑制 GPR68 还能以 Ca2+ 无关的方式减少 AML 细胞中的有氧糖酵解,这表明 GPR68 介导了葡萄糖代谢共生。由于葡萄糖代谢共生以及对有氧糖酵解和细胞呼吸的异质性依赖极大地影响了化疗敏感性,因此抑制 GPR68 能增强一线化疗药物的杀瘤效果,包括针对 OxPhos 的 BCL-2 抑制剂和针对糖酵解的阿糖胞苷(AraC)。与这些体外观察结果一致的是,在接受化疗的急性髓细胞性白血病患者中,较高水平的 GPR68 与较差的临床结果相关。简而言之,GPR68 驱动 Ca2+/CaN 促进生存途径,并介导 AML 细胞中的葡萄糖代谢途径。以 GPR68 为靶点可消灭急性髓细胞白血病细胞并减轻化疗耐药性,因此可将其作为治疗靶点。GPR68的过表达驱动了Ca2+/CaN促生存途径,并介导了AML细胞中的葡萄糖代谢共生,这表明GPR68在AML中具有诊断和治疗潜力。(GPR68,G质子偶联受体68;PLCβ,磷脂酶C beta;CaN,钙神经蛋白;IDH,异柠檬酸脱氢酶;HIF-1α,缺氧诱导因子α亚基;GLUT1,葡萄糖转运体1型;HK-1,六磷酸酶1)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
12.30
自引率
0.00%
发文量
218
审稿时长
32 days
期刊介绍:
BBA Molecular Basis of Disease addresses the biochemistry and molecular genetics of disease processes and models of human disease. This journal covers aspects of aging, cancer, metabolic-, neurological-, and immunological-based disease. Manuscripts focused on using animal models to elucidate biochemical and mechanistic insight in each of these conditions, are particularly encouraged. Manuscripts should emphasize the underlying mechanisms of disease pathways and provide novel contributions to the understanding and/or treatment of these disorders. Highly descriptive and method development submissions may be declined without full review. The submission of uninvited reviews to BBA - Molecular Basis of Disease is strongly discouraged, and any such uninvited review should be accompanied by a coverletter outlining the compelling reasons why the review should be considered.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


