WATER versus WATER II 5-year update: Comparing Aquablation therapy for benign prostatic hyperplasia in 30–80-cm3 and 80–150-cm3 prostates
Abstract
Objective
This study aims to compare the long-term outcomes of Aquablation for small-to-moderate (30–80 cm3) prostates with the outcomes for large (80–150 cm3) prostates at 5-year follow up.
Methods
The Waterjet Ablation Therapy for Endoscopic Resection of Prostate Tissue (WATER; NCT02505919) is a prospective, double-blind, international clinical trial encompassing 116 patients, examining Aquablation versus transurethral resection of the prostate (TURP) for LUTS/BPH in prostates sized between 30 and 80 cm3. In parallel, WATER II (W-II; NCT03123250), a prospective, multicentre, single-arm international clinical trial, explores Aquablation outcomes in prostates ranging from 80 to 150 cm3. Baseline parameters and 60-month outcomes were scrutinized using statistical analyses, including Students' t test, Wilcoxon tests for continuous variables, and Fisher's test for binary variables.
Results
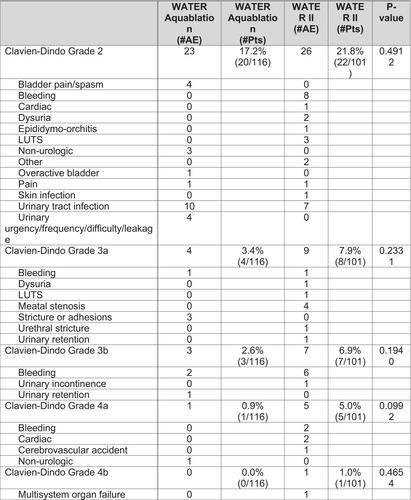
There is a significant improvement in International Prostate Symptom Score (IPSS) from baseline to 60 months in both WATER (22.9 to 7.0) and WATER II (23.2 to 6.8) (P = 0.852). Urinary flow rate (Qmax) increased in both groups from baseline to 60 months (WATER: 9.4 to 17.3 cc/s; WATER II: 8.7 to 17.1 cc/s) (P = 0.933). Immediate and sustained enhancements were observed in IPSS and Qmax. At 5 years, a notable percentage of patients in both groups were BPH medication-free (WATER: 99%; WATER II: 94%) (P = 0.0517) and free from surgical retreatment (WATER: 95%; WATER II: 97%) (P = 0.508).
Conclusions
The 5-year follow-up affirms that Aquablation therapy exhibits sustained outcomes, minimal irreversible complications, and low retreatment rates for treating LUTS/BPH, irrespective of prostate volume ranging from 30 to 150 cm3.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


