A novel regulatory axis of MSI2-AGO2/miR-30a-3p-CGRRF1 drives cancer chemoresistance by upregulating the KRAS/ERK pathway
IF 4.8
2区 医学
Q1 Biochemistry, Genetics and Molecular Biology
引用次数: 0
Abstract
The KRAS/ERK pathway is crucial in cancer progression and chemotherapy resistance, yet its upstream regulatory mechanism remains elusive. We identified MSI2 as a new promoter of chemotherapy resistance in cancers. MSI2 directly binds to a specific class of mature miRNAs by recognizing the 'UAG' motif and interacts with the essential effector AGO2, highlighting MSI2 as a novel regulatory factor within the miRNA pathway. Specifically, MSI2 recruits UAG-miRNA miR-30a-3p to facilitate its loading onto AGO2, efficiently inhibiting the expression of CGRRF1. Further analysis reveals that CGRRF1 functions as a new ubiquitin E3 ligase for KRAS, mediating the ubiquitination and proteasome degradation of KRAS. Consequently, a novel regulatory axis involving MSI2-AGO2/miR-30a-3p-CGRRF1 positively regulates the KRAS/ERK pathway. Remarkably, platinum-based chemotherapy drugs significantly enhance the levels of phosphorylated ERK1/2 (p-ERK1/2) in cancer cells, and the EGFR inhibitor Gefitinib also increases p-ERK1/2 levels in Gefitinib-resistant cancer cells. Combining small-molecule inhibitors targeting MSI2, such as Ro 08-2750, efficiently alleviated chemoresistance in tumor cells exposed to Platinum and Gefitinib. These findings suggest that MSI2 could be a novel therapeutic target for developing strategies to counteract cancer resistance to treatment.
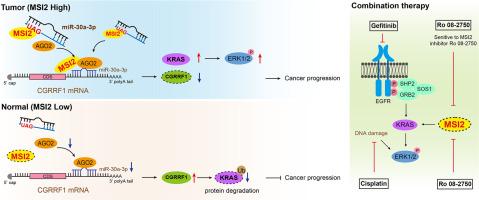
MSI2-AGO2/miR-30a-3p-CGRRF1的新型调控轴通过上调KRAS/ERK通路驱动癌症化疗抗性。
KRAS/ERK通路在癌症进展和化疗耐药性中至关重要,但其上游调控机制仍然难以捉摸。我们发现 MSI2 是癌症化疗耐药性的新启动子。MSI2通过识别 "UAG "基序直接与一类特定的成熟miRNA结合,并与重要的效应因子AGO2相互作用,从而凸显了MSI2是miRNA通路中的一种新型调控因子。具体地说,MSI2 会吸引 UAG-miRNA miR-30a-3p 加载到 AGO2 上,从而有效抑制 CGRRF1 的表达。进一步分析表明,CGRRF1 可作为 KRAS 新的泛素 E3 连接酶,介导 KRAS 的泛素化和蛋白酶体降解。因此,一个涉及 MSI2-AGO2/miR-30a-3p-CGRRF1 的新型调控轴正调控着 KRAS/ERK 通路。值得注意的是,铂类化疗药物会显著提高癌细胞中磷酸化 ERK1/2(p-ERK1/2)的水平,而表皮生长因子受体抑制剂吉非替尼(Gefitinib)也会提高吉非替尼耐药癌细胞中 p-ERK1/2 的水平。联合使用靶向MSI2的小分子抑制剂(如Ro 08-2750),可有效缓解暴露于铂类和吉非替尼的肿瘤细胞的化疗耐药性。这些研究结果表明,MSI2可能是一种新型治疗靶点,可用于开发抗击癌症耐药性的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Neoplasia
医学-肿瘤学
CiteScore
9.20
自引率
2.10%
发文量
82
审稿时长
26 days
期刊介绍:
Neoplasia publishes the results of novel investigations in all areas of oncology research. The title Neoplasia was chosen to convey the journal’s breadth, which encompasses the traditional disciplines of cancer research as well as emerging fields and interdisciplinary investigations. Neoplasia is interested in studies describing new molecular and genetic findings relating to the neoplastic phenotype and in laboratory and clinical studies demonstrating creative applications of advances in the basic sciences to risk assessment, prognostic indications, detection, diagnosis, and treatment. In addition to regular Research Reports, Neoplasia also publishes Reviews and Meeting Reports. Neoplasia is committed to ensuring a thorough, fair, and rapid review and publication schedule to further its mission of serving both the scientific and clinical communities by disseminating important data and ideas in cancer research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


