Nicotine and a positive allosteric modulator of m1 muscarinic receptor increase NMDA/AMPA ratio in the hippocampus and medial prefrontal cortex
IF 4.6
2区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
Chronic nicotine exposure has been shown to improve memory in rodents. However, the molecular mechanism for such an enhancement remains poorly understood. Chronic nicotine exposure increases NMDA/AMPA ratio due to enhanced NMDAR-mediated responses in hippocampal CA1 pyramidal cells and facilitates LTP. Here, we found that the same nicotine treatment increases NMDA/AMPA ratios in parvalbumin-expressing interneurons in the hippocampus and in layer 5 pyramidal cells in the medial prefrontal cortex (mPFC) of male and female rats. To gain further insight into the nicotine-initiated signaling pathway, we used a positive allosteric modulator (PAM) of m1 muscarinic acetylcholine receptor (m1 receptor), VU0453595. We found that chronic VU0453595 treatment mimics the effects of chronic nicotine exposure, causing increased NMDA/AMPA ratio in hippocampal CA1 pyramidal cells and LTP facilitation. Furthermore, chronic exposure to VU0453595 also caused increased NMDA/AMPA ratio in layer 5 pyramidal cells of mPFC. As the PAM only activates m1 receptors when the endogenous agonist acetylcholine (ACh) is present, the findings suggest that the release of ACh from cholinergic neurons is involved in the effect. Thus, chronic nicotine exposure, by increasing ACh release, may stimulate a signaling pathway in various neuron types, which receive cholinergic input and express m1 receptors, leading to the enhancement of NMDAR responses. The nicotine-initiated signaling pathway, in which ACh and m1 receptors are downstream of nicotinic ACh receptor activation, may represent an important cholinergic pathway involved in cognitive function.
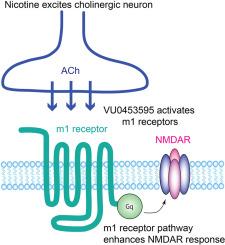
尼古丁和一种m1毒蕈碱受体正异位调节剂会增加海马和内侧前额叶皮层中的NMDA/AMPA比率。
研究表明,长期接触尼古丁可改善啮齿类动物的记忆力。然而,这种改善的分子机制仍然鲜为人知。由于海马CA1锥体细胞中NMDAR介导的反应增强,慢性尼古丁暴露会增加NMDA/AMPA比率,并促进LTP。在这里,我们发现同样的尼古丁处理会增加雄性和雌性大鼠海马中表达parvalbumin的中间神经元以及内侧前额叶皮层(mPFC)第5层锥体细胞中的NMDA/AMPA比率。为了进一步了解尼古丁引发的信号通路,我们使用了一种 m1 毒蕈碱乙酰胆碱受体(m1 受体)的正异位调节剂(PAM)--VU0453595。我们发现,慢性 VU0453595 治疗可模拟慢性尼古丁暴露的效应,导致海马 CA1 锥体细胞中的 NMDA/AMPA 比率增加和 LTP 促进。此外,长期暴露于 VU0453595 还会导致 mPFC 第 5 层锥体细胞中的 NMDA/AMPA 比率增加。由于只有当内源性激动剂乙酰胆碱(ACh)存在时,PAM 才会激活 m1 受体,因此研究结果表明,胆碱能神经元释放的 ACh 参与了这一效应。因此,长期暴露于尼古丁会增加 ACh 的释放,从而刺激接受胆碱能输入并表达 m1 受体的各种类型神经元的信号通路,导致 NMDAR 反应的增强。在尼古丁引发的信号通路中,ACh 和 m1 受体是尼古丁 ACh 受体激活的下游,这可能是参与认知功能的重要胆碱能通路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Neuropharmacology
医学-神经科学
CiteScore
10.00
自引率
4.30%
发文量
288
审稿时长
45 days
期刊介绍:
Neuropharmacology publishes high quality, original research and review articles within the discipline of neuroscience, especially articles with a neuropharmacological component. However, papers within any area of neuroscience will be considered. The journal does not usually accept clinical research, although preclinical neuropharmacological studies in humans may be considered. The journal only considers submissions in which the chemical structures and compositions of experimental agents are readily available in the literature or disclosed by the authors in the submitted manuscript. Only in exceptional circumstances will natural products be considered, and then only if the preparation is well defined by scientific means. Neuropharmacology publishes articles of any length (original research and reviews).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


