Variable glucagon metabolic actions in diverse mouse models of obesity and type 2 diabetes
IF 7
2区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
Objective
The study aimed to investigate the effects of glucagon on metabolic pathways in mouse models of obesity, fatty liver disease, and type 2 diabetes (T2D) to determine the extent and variability of hepatic glucagon resistance in these conditions.
Methods
We investigated glucagon's effects in mouse models of fatty liver disease, obesity, and type 2 diabetes (T2D), including male BKS-db/db, high-fat diet-fed, and western diet-fed C57Bl/6 mice. Glucagon tolerance tests were performed using the selective glucagon receptor agonist acyl-glucagon (IUB288). Blood glucose, serum and liver metabolites include lipids and amino acids were measured. Additionally, liver protein expression related to glucagon signalling and a comprehensive liver metabolomics were performed.
Results
Western diet-fed mice displayed impaired glucagon response, with reduced blood glucose and PKA activation. In contrast, high-fat diet-fed and db/db mice maintained normal glucagon sensitivity, showing significant elevations in blood glucose and phospho-PKA motif protein expression. Acyl-glucagon treatment also lowered liver alanine and histidine levels in high-fat diet-fed mice, but not in western diet-fed mice. Additionally, some amino acids, such as methionine, were increased by acyl-glucagon only in chow diet control mice. Despite normal glucagon sensitivity in PKA signalling, db/db mice had a distinct metabolomic response, with acyl-glucagon significantly altering 90 metabolites in db/+ mice but only 42 in db/db mice, and classic glucagon-regulated metabolites, such as cyclic adenosine monophosphate (cAMP), being less responsive in db/db mice.
Conclusions
The study reveals that hepatic glucagon resistance in obesity and T2D is complex and not uniform across metabolic pathways, underscoring the complexity of glucagon action in these conditions.
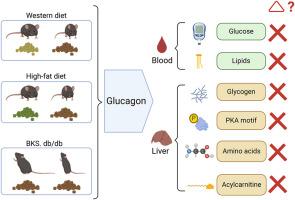
胰高血糖素在不同肥胖症和 2 型糖尿病小鼠模型中的代谢作用各不相同。
研究目的本研究旨在调查胰高血糖素对肥胖、脂肪肝和2型糖尿病(T2D)小鼠模型代谢途径的影响,以确定在这些情况下肝脏胰高血糖素抵抗的程度和可变性:我们研究了胰高血糖素对脂肪肝、肥胖和2型糖尿病(T2D)小鼠模型的影响,包括雄性BKS-db/db、高脂饮食喂养和西式饮食喂养的C57Bl/6小鼠。使用选择性胰高血糖素受体激动剂酰基胰高血糖素(IUB288)进行胰高血糖素耐受试验。对血糖、血清和肝脏代谢物(包括脂类和氨基酸)进行了测定。此外,还进行了与胰高血糖素信号相关的肝脏蛋白质表达和全面的肝脏代谢组学研究:结果:西式饮食喂养的小鼠显示出胰高血糖素反应受损,血糖降低,PKA活化。相比之下,高脂饮食喂养的小鼠和 db/db 小鼠对胰高血糖素的敏感性保持正常,血糖和磷酸-PKA 矩阵蛋白表达显著升高。酰基胰高血糖素处理还能降低高脂饮食喂养小鼠肝脏丙氨酸和组氨酸的水平,但不能降低西式饮食喂养小鼠肝脏丙氨酸和组氨酸的水平。此外,酰基胰高血糖素还能增加某些氨基酸的含量,如蛋氨酸,但只有在低脂饮食对照组小鼠中才会出现这种情况。尽管胰高血糖素对PKA信号的敏感性正常,但db/db小鼠的代谢组学反应却截然不同,酰基胰高血糖素可显著改变db/+小鼠的90种代谢物,但在db/db小鼠中仅有42种代谢物发生了改变,而胰高血糖素调节的典型代谢物,如环磷酸腺苷(cAMP),在db/db小鼠中反应较小:结论:该研究揭示了肥胖症和T2D的肝脏胰高血糖素抵抗是复杂的,而且在不同的代谢途径中并不一致,这凸显了胰高血糖素在这些情况下作用的复杂性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
14.50
自引率
2.50%
发文量
219
审稿时长
43 days
期刊介绍:
Molecular Metabolism is a leading journal dedicated to sharing groundbreaking discoveries in the field of energy homeostasis and the underlying factors of metabolic disorders. These disorders include obesity, diabetes, cardiovascular disease, and cancer. Our journal focuses on publishing research driven by hypotheses and conducted to the highest standards, aiming to provide a mechanistic understanding of energy homeostasis-related behavior, physiology, and dysfunction.
We promote interdisciplinary science, covering a broad range of approaches from molecules to humans throughout the lifespan. Our goal is to contribute to transformative research in metabolism, which has the potential to revolutionize the field. By enabling progress in the prognosis, prevention, and ultimately the cure of metabolic disorders and their long-term complications, our journal seeks to better the future of health and well-being.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


