Time-restricted feeding prevents memory impairments induced by obesogenic diet consumption, via hippocampal thyroid hormone signaling
IF 6.6
2区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
Objective
The early consumption of calorie-rich diet disrupts circadian rhythms and has adverse effects on memory, yet the effects of time-restricted feeding (TRF) and the underlying molecular mechanisms are unknown. Here, we set out to identify the behavioral and molecular circadian rhythms disruptions generated by juvenile obesogenic diet consumption and their restoration by TRF in male mice.
Methods
Metabolic rhythms were measured by indirect calorimetry and memory performances by behavioral tasks. Hippocampal translatome (pS6_TRAP), enrichment and co-regulated gene network analyses were conducted to identify the molecular pathways involved in memory impairments and their restoration by TRF. Differential exon usage analyses, mass spectrometry and pharmacological intervention were used to confirm thyroid hormone signaling involvement.
Results
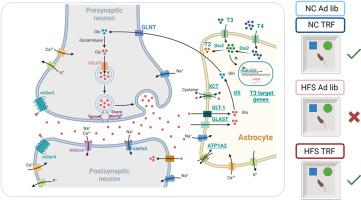
We show that four weeks of TRF restore the rhythmicity of metabolic parameters and prevents memory impairments in mice fed a high fat-high sucrose (HFS) diet since weaning, independently of body fat levels. Hippocampal translatome and differential exon usage analyses indicate that impaired memory of mice under ad libitum HFS diet is accompanied by reduced thyroid hormone signaling and altered expression of astrocytic genes regulating glutamate neurotransmission. TRF restored the diurnal expression variation of part of these genes and intra-hippocampal infusion of T3, the active form of thyroid hormone, rescues memory performances and astrocytic gene expression of ad libitum HFS diet-fed mice.
Conclusions
Thus, thyroid hormones contribute to the TRF positive effects on both metabolism and memory in mice fed an obesogenic diet, highlighting this nutritional approach as a powerful tool in addressing obesity brain comorbidities and paving the way for further mechanistic studies on hippocampal thyroid signaling.
限时进食可通过海马甲状腺激素信号传导预防肥胖饮食引起的记忆损伤。
目的:早期摄入高热量饮食会扰乱昼夜节律并对记忆产生不利影响,但限时喂养(TRF)的影响及其潜在的分子机制尚不清楚。在此,我们试图确定幼年肥胖饮食对雄性小鼠行为和分子昼夜节律的干扰,以及通过TRF恢复这些干扰:方法:代谢节律通过间接热量计测量,记忆表现通过行为任务测量。方法:通过间接热量计测量代谢节律,通过行为任务测量记忆表现,进行海马转译组(pS6_TRAP)、富集和共调基因网络分析,以确定参与记忆损伤的分子通路,以及通过TRF恢复记忆的分子通路。差异外显子使用分析、质谱分析和药物干预被用来证实甲状腺激素信号的参与:结果:我们发现,对自断奶起就以高脂肪-高蔗糖(HFS)饮食喂养的小鼠来说,四周的TRF可恢复代谢参数的节律性并防止记忆损伤,而与体脂水平无关。海马转位组和差异外显子使用分析表明,小鼠在自由摄入高脂高蔗糖饮食的情况下记忆力受损,与甲状腺激素信号传导减少和调节谷氨酸神经传导的星形胶质细胞基因表达改变有关。TRF可恢复这些基因中部分基因的昼夜表达变化,而在海马内注入甲状腺激素的活性形式T3,可挽救自由摄入HFS饮食的小鼠的记忆表现和星形胶质细胞基因表达:因此,甲状腺激素有助于TRF对肥胖饮食喂养小鼠的新陈代谢和记忆力产生积极影响,凸显了这种营养方法是解决肥胖脑合并症的有力工具,并为进一步开展海马甲状腺信号转导的机理研究铺平了道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
14.50
自引率
2.50%
发文量
219
审稿时长
43 days
期刊介绍:
Molecular Metabolism is a leading journal dedicated to sharing groundbreaking discoveries in the field of energy homeostasis and the underlying factors of metabolic disorders. These disorders include obesity, diabetes, cardiovascular disease, and cancer. Our journal focuses on publishing research driven by hypotheses and conducted to the highest standards, aiming to provide a mechanistic understanding of energy homeostasis-related behavior, physiology, and dysfunction.
We promote interdisciplinary science, covering a broad range of approaches from molecules to humans throughout the lifespan. Our goal is to contribute to transformative research in metabolism, which has the potential to revolutionize the field. By enabling progress in the prognosis, prevention, and ultimately the cure of metabolic disorders and their long-term complications, our journal seeks to better the future of health and well-being.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


