Hepatoprotective and neuroprotective effects of quinacrine against bile duct ligation-induced hepatic encephalopathy in rats: Role of bone morphogenetic proteins signaling
IF 5.2
2区 医学
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
Abstract
Aims
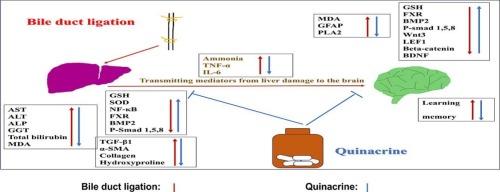
This study aimed to assess the potential protective effect of quinacrine, an FDA approved antimalarial drug with reported anti-inflammatory effects, on hepatic encephalopathy (HE) in a bile duct ligation (BDL) experimental model and to investigate the mechanisms responsible for this effect, namely those associated with the liver-brain axis, particularly, bone morphogenetic protein 2 (BMP2) signaling.
Materials and methods
Five groups of rats were selected at random: sham, BDL, (BDL+ quinacrine 5), (BDL+ quinacrine 10), and (quinacrine 10 + sham). Daily Intraperitoneal (I.P.) administration of quinacrine was initiated on the surgery day and continued for 28 days.
Key findings
Results showed that rats that underwent BDL exhibited marked elevation of serum liver enzymes, ammonia, total bilirubin, together with oxidative stress, inflammation, dysregulated farnesoid x receptor (FXR), dysregulated BMP2 signaling and escalated fibrotic markers indicating hepatotoxicity, cholestasis and fibrosis. Besides, neurotoxicity was detected as manifested by cognitive deficits and dysregulation of hippocampal FXR, BMP2 signaling, WNT3A signaling, brain derived neurotrophic factor (BDNF), phospholipase A2 (PLA2) and glial fibrillary acidic protein (GFAP). In contrast, co-treatment with quinacrine mitigated BDL-induced hepatotoxicity, cholestasis, fibrosis, and neurotoxicity. Notably, quinacrine improved learning and memory and restored FXR, BMP2 signaling in the liver and hippocampus. In addition, quinacrine restored hippocampal WNT3A signaling, BDNF, whereas it downregulated expression of hippocampal PLA2 and GFAP.
Significance
These findings demonstrated implication of BMP2 signaling in the molecular process of BDL-induced HE and proposed that quinacrine has potential hepatoprotective and neuroprotective properties against HE.
喹吖啶对胆管结扎诱导的大鼠肝性脑病的肝保护和神经保护作用:骨形态发生蛋白信号传导的作用
目的:本研究旨在评估喹吖啶(美国食品药物管理局批准的一种抗疟药物,据报道具有抗炎作用)对胆管结扎(BDL)实验模型中肝性脑病(HE)的潜在保护作用,并研究产生这种作用的机制,即与肝-脑轴相关的机制,特别是骨形态发生蛋白2(BMP2)信号转导:随机选取五组大鼠:假、BDL、(BDL+喹吖啶 5)、(BDL+喹吖啶 10)和(喹吖啶 10 + 假)。手术当天开始每日腹腔注射喹吖啶,并持续28天:结果显示,接受 BDL 的大鼠表现出血清肝酶、氨、总胆红素明显升高,同时伴有氧化应激、炎症、类雌激素 x 受体(FXR)失调、BMP2 信号传导失调和纤维化标志物升高,表明存在肝毒性、胆汁淤积和纤维化。此外,还发现了神经毒性,表现为认知障碍和海马 FXR、BMP2 信号、WNT3A 信号、脑源性神经营养因子(BDNF)、磷脂酶 A2(PLA2)和神经胶质纤维酸性蛋白(GFAP)失调。相比之下,联合使用奎那新可减轻 BDL 诱导的肝毒性、胆汁淤积、肝纤维化和神经毒性。值得注意的是,奎那新能改善学习和记忆,恢复肝脏和海马中的 FXR 和 BMP2 信号传导。此外,奎那新还能恢复海马WNT3A信号和BDNF,同时下调海马PLA2和GFAP的表达:这些发现证明了BMP2信号在BDL诱导的肝癌分子过程中的作用,并提出了奎那新对肝癌具有潜在的保肝和神经保护作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Life sciences
医学-药学
CiteScore
12.20
自引率
1.60%
发文量
841
审稿时长
6 months
期刊介绍:
Life Sciences is an international journal publishing articles that emphasize the molecular, cellular, and functional basis of therapy. The journal emphasizes the understanding of mechanism that is relevant to all aspects of human disease and translation to patients. All articles are rigorously reviewed.
The Journal favors publication of full-length papers where modern scientific technologies are used to explain molecular, cellular and physiological mechanisms. Articles that merely report observations are rarely accepted. Recommendations from the Declaration of Helsinki or NIH guidelines for care and use of laboratory animals must be adhered to. Articles should be written at a level accessible to readers who are non-specialists in the topic of the article themselves, but who are interested in the research. The Journal welcomes reviews on topics of wide interest to investigators in the life sciences. We particularly encourage submission of brief, focused reviews containing high-quality artwork and require the use of mechanistic summary diagrams.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


