Magnolol preserves the integrity of the intestinal epithelial barrier and mitigates intestinal injury through activation of PPAR γ in COPD rat
IF 4.8
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Ethnopharmacological relevance
Magnolia officinalis Rehder & E.H. Wilson is traditionally used in the treatment of gastrointestinal disorders, diarrhea, and cough. Its main active ingredient, magnolol, exhibits protective effects on the lungs and gastrointestinal tract, including the inhibition of inflammation in these organs.
Aim of the study
This work aims to explore the molecular mechanism by which magnolol suppressed Chronic obstructive pulmonary disease (COPD) intestinal damage by improving the intestinal epithelial barrier.
Materials and methods
The study focused on investigating the mitigation effect of magnolol on intestinal injury and epithelial barrier in a COPD rat. Caco-2 cells were induced with TNF-α or IL-1β to establish the barrier injury model in order to explore the direct protective effect of magnolol on the intestinal barrier and elucidate the molecular mechanism by which it activates peroxisome proliferators-activated receptors-γ (PPARγ).
Results
Magnolol significantly improves pulmonary function and tissue damage in COPD rats by inhibiting inflammation, protease imbalance, and oxidative stress. It also suppresses colon tissue damage and inflammation, and protects colon epithelial barrier function by suppressing the decline of tight junction proteins, reducing colon epithelial permeability. In Caco-2 cells, magnolol directly reduces monolayer permeability, increases TEER, and upregulates tight junction protein expression induced by TNF-α or IL-1β. Drug Affinity Responsive Target Stability (DARTS) and thermal shift assays show that magnolol effectively binds to SRC, activating PPARγ signaling in Caco-2 cells and colon tissues of COPD rats. Furthermore, magnolol enhances the binding of PPARγ and RXRα, promoting their activation and entry into the nucleus. The PPARγ inhibitor GW9662 can reverse the effects of magnolol on PPARγ activation and tight junction protein upregulation in IL-1β or TNF-α induced Caco-2 cells.
Conclusions
This work demonstrates that magnolol enhances lung and intestinal functions in COPD rats, and elucidates its mechanism of action in protecting the intestinal epithelial barrier by activating PPARγ.
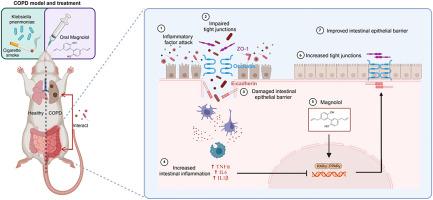
厚朴酚通过激活 PPAR γ 保护慢性阻塞性肺病大鼠肠上皮屏障的完整性并减轻肠道损伤。
民族药理学意义:厚朴(Magnolia officinalis Rehder & E.H. Wilson)传统上用于治疗胃肠道疾病、腹泻和咳嗽。其主要活性成分木兰醇对肺部和胃肠道具有保护作用,包括抑制这些器官的炎症:研究目的:本研究旨在探索麦格诺尔通过改善肠上皮屏障抑制慢性阻塞性肺病(COPD)肠道损伤的分子机制:研究重点是探讨马格诺尔对慢性阻塞性肺病大鼠肠道损伤和上皮屏障的缓解作用。用TNF-α或IL-1β诱导Caco-2细胞建立屏障损伤模型,以探讨马格诺尔对肠道屏障的直接保护作用,并阐明其激活过氧化物酶体增殖物激活受体-γ(PPARγ)的分子机制:结果:木酚醇通过抑制炎症、蛋白酶失衡和氧化应激,明显改善慢性阻塞性肺疾病大鼠的肺功能和组织损伤。它还能抑制结肠组织损伤和炎症,并通过抑制紧密连接蛋白的下降保护结肠上皮屏障功能,降低结肠上皮的通透性。在 Caco-2 细胞中,magnolol 可直接降低单层渗透性,增加 TEER,并上调 TNF-α 或 IL-1β 诱导的紧密连接蛋白表达。药物亲和力反应靶点稳定性(DARTS)和热转移试验表明,奥美洛尔能有效地与SRC结合,激活Caco-2细胞和慢性阻塞性肺病大鼠结肠组织中的PPARγ信号传导。此外,magnolol 还能增强 PPARγ 和 RXRα 的结合,促进其活化并进入细胞核。PPARγ抑制剂GW9662可逆转magnolol对IL-1β或TNF-α诱导的Caco-2细胞中PPARγ活化和紧密连接蛋白上调的影响:本研究表明,麦格诺尔能增强慢性阻塞性肺疾病大鼠的肺功能和肠功能,并阐明了其通过激活 PPARγ 保护肠上皮屏障的作用机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of ethnopharmacology
医学-全科医学与补充医学
CiteScore
10.30
自引率
5.60%
发文量
967
审稿时长
77 days
期刊介绍:
The Journal of Ethnopharmacology is dedicated to the exchange of information and understandings about people''s use of plants, fungi, animals, microorganisms and minerals and their biological and pharmacological effects based on the principles established through international conventions. Early people confronted with illness and disease, discovered a wealth of useful therapeutic agents in the plant and animal kingdoms. The empirical knowledge of these medicinal substances and their toxic potential was passed on by oral tradition and sometimes recorded in herbals and other texts on materia medica. Many valuable drugs of today (e.g., atropine, ephedrine, tubocurarine, digoxin, reserpine) came into use through the study of indigenous remedies. Chemists continue to use plant-derived drugs (e.g., morphine, taxol, physostigmine, quinidine, emetine) as prototypes in their attempts to develop more effective and less toxic medicinals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


