Melatonin attenuates degenerative disc degression by downregulating DLX5 via the TGF/Smad2/3 pathway in nucleus pulposus cells
Abstract
Background
Intervertebral disc degeneration (IVDD) is the leading cause of low back pain, and apoptosis plays a key role in its pathogenesis. Distal-less homeobox 5 (Dlx5) has been reported to induce cell apoptosis. Melatonin, as a powerful antiapoptotic agent, has been widely reported.
Aim
This study aimed to investigate the role of DLX5 in the pathogenesis of IVDD and the potential therapeutic role of melatonin in targeting DLX5 in IVDD.
Materials & Methods
Western blotting, RT–qPCR, immunohistochemistry, si-DLX5, Ex-DLX5, flow cytometry, and immunofluorescence were used to examine the regulatory effect of DLX5 on apoptosis. Therapeutic efficacy was assessed by the intraperitoneal injection of melatonin into IVDD mice.
Results
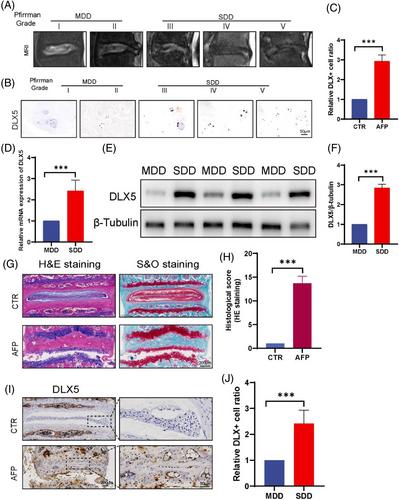
The expression level of DLX5 is significantly increased in IVDD, and the expression levels were positively correlated with the grade of IVDD. DLX5 was significantly upregulated in TNF-α-induced degenerative NP cells. Degenerative NP cells transfected with si-DLX5 exhibited significantly less apoptosis than control cells. Melatonin significantly alleviated IVDD in surgically induced IVDD model mice.
Discussion
The results revealed that the expression of DLX5 was positively correlated with the severity of IVDD and that melatonin ameliorated DLX5-induced apoptosis and extracellular matrix imbalance by inhibiting the TGF-β/Smad signaling pathway. This study may provide therapeutic strategies to alleviate inflammation-induced apoptosis IVDD-associated inflammation-induced apoptosis.
Conclusion
DLX5 plays an important role in IVDD progression by promoting apoptosis, and melatonin represents a promising therapeutic strategy for alleviating IVDD-associated inflammation and apoptosis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


