Efficacy and safety of dostarlimab in combination with chemotherapy in patients with dMMR/MSI-H primary advanced or recurrent endometrial cancer in a phase 3, randomized, placebo-controlled trial (ENGOT-EN6-NSGO/GOG-3031/RUBY)
IF 4.5
2区 医学
Q1 OBSTETRICS & GYNECOLOGY
引用次数: 0
Abstract
Objectives
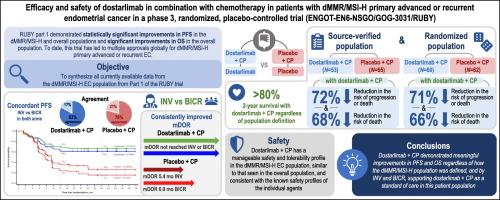
Part 1 of the RUBY trial (NCT03981796) demonstrated improved survival in patients with primary advanced or recurrent endometrial cancer (EC) treated with dostarlimab plus carboplatin-paclitaxel versus placebo plus carboplatin-paclitaxel. Here, we examine additional efficacy and safety data from patients with mismatch repair deficient/microsatellite instability-high (dMMR/MSI-H) EC in the RUBY trial.
Methods
Patients were randomized 1:1 to dostarlimab 500 mg or placebo plus carboplatin-paclitaxel every 3 weeks for 6 cycles followed by dostarlimab or placebo every 6 weeks for up to 3 years. In the dMMR/MSI-H population of RUBY Part 1, analysis of progression-free survival by investigator assessment compared with blinded independent central review, sensitivity analyses of the source-verified population compared with the randomized population, and analysis of safety in this population were completed.
Results
In total, 118 patients with dMMR/MSI-H were enrolled in the RUBY trial (53, dostarlimab arm; 65, placebo arm). At the first interim analysis, a 72% reduction in the risk of progression or death (P < 0.0001) was seen with dostarlimab plus carboplatin-paclitaxel by investigator assessment per Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1), which was consistent with blinded independent central review per RECIST v1.1. Likewise, sensitivity analyses of the source-verified dMMR/MSI-H population compared with the randomized dMMR/MSI-H population were consistent for progression-free survival and overall survival. Safety results seen in the dMMR/MSI-H population were similar to those previously reported for the overall population.
Conclusions
All primary and secondary efficacy assessments demonstrate the consistent benefit of dostarlimab plus carboplatin-paclitaxel. The improvements seen in survival and the manageable safety profile support the favorable benefit–risk profile for dostarlimab plus carboplatin-paclitaxel in patients with dMMR/MSI-H primary advanced or recurrent EC.
在一项 3 期随机安慰剂对照试验(ENGOT-EN6-NSGO/GOG-3031/RUBY)中,多司替雷单抗联合化疗对 dMMR/MSI-H 原发性晚期或复发性子宫内膜癌患者的疗效和安全性。
研究目的RUBY试验(NCT03981796)第一部分显示,多司他单抗联合卡铂-紫杉醇与安慰剂联合卡铂-紫杉醇治疗原发性晚期或复发性子宫内膜癌(EC)患者的生存率有所提高。在此,我们研究了RUBY试验中错配修复缺陷/微卫星不稳定性高(dMMR/MSI-H)EC患者的其他疗效和安全性数据:患者按1:1比例随机分配到多司他利单抗500毫克或安慰剂加卡铂-紫杉醇治疗,每3周1次,共6个周期,然后再分配到多司他利单抗或安慰剂治疗,每6周1次,共3年。在RUBY第一部分的dMMR/MSI-H人群中,完成了研究者评估与盲法独立中央审查的无进展生存期分析、来源验证人群与随机人群的敏感性分析以及该人群的安全性分析:RUBY试验共招募了118名dMMR/MSI-H患者(多司他利单抗组53人;安慰剂组65人)。在第一次中期分析中,病情恶化或死亡风险降低了72%(P结论):所有主要和次要疗效评估结果都表明,多斯他利单抗联合卡铂-紫杉醇治疗具有持续的疗效。生存期的改善和可控的安全性支持了多斯他利单抗联合卡铂-紫杉醇治疗dMMR/MSI-H原发性晚期或复发性EC患者的有利获益-风险特征。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Gynecologic oncology
医学-妇产科学
CiteScore
8.60
自引率
6.40%
发文量
1062
审稿时长
37 days
期刊介绍:
Gynecologic Oncology, an international journal, is devoted to the publication of clinical and investigative articles that concern tumors of the female reproductive tract. Investigations relating to the etiology, diagnosis, and treatment of female cancers, as well as research from any of the disciplines related to this field of interest, are published.
Research Areas Include:
• Cell and molecular biology
• Chemotherapy
• Cytology
• Endocrinology
• Epidemiology
• Genetics
• Gynecologic surgery
• Immunology
• Pathology
• Radiotherapy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


