Intracellular delivery of a phospholamban-targeting aptamer using cardiomyocyte-internalizing aptamers
IF 4.2
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
The sarco (endo)plasmic reticulum Ca2+-ATPase 2a (SERCA2a)–phospholamban (PLN) system within the sarcoplasmic reticulum is crucial for regulating intracellular Ca2+ cycling in ventricular cardiomyocytes. Given that impaired Ca2+ cycling is associated with heart failure, modulating SERCA2a activity represents a promising therapeutic strategy. Previously, we engineered an RNA aptamer (Apt30) that binds to PLN, thereby activating SERCA2a by alleviating PLN's inhibitory effect. However, Apt30 alone cannot reach intracellular PLN, necessitating the development of a mechanism for its specific internalization into cardiomyocytes.
Using the systematic evolution of ligands by exponential enrichment (SELEX) method, we isolated RNA aptamers capable of internalizing into cardiomyocytes. These aptamers demonstrated sub-micromolar EC50 values for cardiomyocyte internalization and exhibited significantly reduced activity against various non-myocardial cells, highlighting their specificity for cardiomyocytes. Moreover, some of these cardiomyocyte-internalizing aptamers could be linked to Apt30 as a single RNA strand without compromising their internalization efficacy. Supplementing the culture medium with these hybrid aptamers enhanced Ca2+ transients and contractile function in rat cardiomyocytes. These findings provide critical insights for developing novel therapeutics directly acting on PLN in cardiomyocytes, potentially compensating for the disadvantages of conventional methods that involve viral vector-mediated intracellular transduction or alterations in endogenous protein expression.
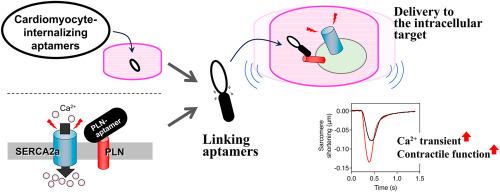
使用心肌细胞内化适配体在细胞内输送磷脂酰胆碱靶向适配体。
肌质网内的肌质网 Ca2+-ATPase 2a(SERCA2a)-磷脂澜班(PLN)系统对于调节心室心肌细胞内的 Ca2+ 循环至关重要。鉴于 Ca2+ 循环受损与心力衰竭有关,调节 SERCA2a 的活性是一种很有前景的治疗策略。此前,我们设计了一种能与 PLN 结合的 RNA 合体(Apt30),从而通过减轻 PLN 的抑制作用来激活 SERCA2a。然而,单靠 Apt30 无法到达细胞内的 PLN,因此有必要开发一种机制,使其特异性地内化到心肌细胞中。利用指数富集配体的系统进化(SELEX)方法,我们分离出了能够内化到心肌细胞中的 RNA 合体。这些适配体对心肌细胞内化的 EC50 值达到亚微摩级,对各种非心肌细胞的活性明显降低,突出了它们对心肌细胞的特异性。此外,其中一些心肌细胞内化适配体可以作为单条 RNA 链与 Apt30 连接,而不会影响其内化功效。在培养基中添加这些混合适配体能增强大鼠心肌细胞的钙离子瞬态和收缩功能。这些发现为开发直接作用于心肌细胞中 PLN 的新型疗法提供了重要启示,有可能弥补病毒载体介导的细胞内转导或改变内源性蛋白表达的传统方法的缺点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.00
自引率
0.00%
发文量
572
审稿时长
34 days
期刊介绍:
The European Journal of Pharmacology publishes research papers covering all aspects of experimental pharmacology with focus on the mechanism of action of structurally identified compounds affecting biological systems.
The scope includes:
Behavioural pharmacology
Neuropharmacology and analgesia
Cardiovascular pharmacology
Pulmonary, gastrointestinal and urogenital pharmacology
Endocrine pharmacology
Immunopharmacology and inflammation
Molecular and cellular pharmacology
Regenerative pharmacology
Biologicals and biotherapeutics
Translational pharmacology
Nutriceutical pharmacology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


