Insight into the molecular mechanism of anti-breast cancer therapeutic potential of substituted salicylidene-based compounds using cell-based assays and molecular docking studies
IF 4.2
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Targeting oxidative stress and inflammatory signaling pathways is an effective cancer prevention and therapy approach. The mechanism of action of synthesized salicylidene-based compounds was investigated in regulating key molecular targets of breast cancer development. Compounds (1), (4), (5), and (7) were found to be more cytotoxic to MCF-7 and 4T1 cells compared to non-cancerous Chang liver cells, while these compounds were cytotoxic to MDA-MB-231 cells, but with poor selectivity. The colony formation assay indicated that bioactive compounds induced significant damage to breast cancer cells, as observed by a reduction in the number of colonies compared to control cells. By inducing a concentration and time-dependent increase of luminescence and fluorescence of phosphatidylserine, and activating the expression of caspases-3, -7, -8, -9 in breast cancer cells, (1) and (7) have shown to induce caspase-dependent apoptosis. The downregulation of NF-kB-p65 and an upregulation of TP53 expression after exposure to bioactive compounds, demonstrated the suppression of two key targets of breast cancer development. Molecular docking studies revealed that selected protein targets strongly interact with bioactive compounds, and the estimated inhibition constants (Ki) of JAK2, STAT3, COX-2, HPV31 E6, EGFR1, TP53, and PARP1 were significantly decreased compared to acetylsalicylic acid. This could be a clear indication that these protein targets are implicated with antiproliferative efficacy, thereby warranting the potential of (1) and (7) to be used as anti-breast cancer drug candidates.
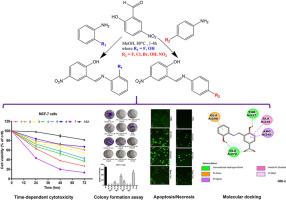
利用细胞分析和分子对接研究,深入了解取代的水杨醛基化合物抗乳腺癌治疗潜力的分子机制。
针对氧化应激和炎症信号通路是一种有效的癌症预防和治疗方法。研究人员探讨了合成的水杨醛基化合物在调节乳腺癌发展关键分子靶点方面的作用机制。研究发现,与非癌变的 Chang 肝细胞相比,化合物(1)、(4)、(5)和(7)对 MCF-7 和 4T1 细胞具有更强的细胞毒性,而这些化合物对 MDA-MB-231 细胞具有细胞毒性,但选择性较差。菌落形成试验表明,生物活性化合物对乳腺癌细胞有明显的损伤作用,与对照细胞相比,菌落数量减少。通过诱导浓度和时间依赖性的磷脂酰丝氨酸发光和荧光增加,以及激活乳腺癌细胞中 caspase-3、-7、-8、-9 的表达,(1) 和 (7) 被证明能诱导 caspase 依赖性凋亡。暴露于生物活性化合物后,NF-kB-p65 的表达下调,TP53 的表达上调,表明乳腺癌发展的两个关键靶点受到抑制。分子对接研究显示,选定的蛋白质靶点与生物活性化合物有强烈的相互作用,与乙酰水杨酸相比,JAK2、STAT3、COX-2、HPV31 E6、表皮生长因子受体1、TP53和PARP1的估计抑制常数(Ki)明显降低。这清楚地表明,这些蛋白靶点与抗增殖功效有关,因此(1)和(7)有可能被用作抗乳腺癌候选药物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.00
自引率
0.00%
发文量
572
审稿时长
34 days
期刊介绍:
The European Journal of Pharmacology publishes research papers covering all aspects of experimental pharmacology with focus on the mechanism of action of structurally identified compounds affecting biological systems.
The scope includes:
Behavioural pharmacology
Neuropharmacology and analgesia
Cardiovascular pharmacology
Pulmonary, gastrointestinal and urogenital pharmacology
Endocrine pharmacology
Immunopharmacology and inflammation
Molecular and cellular pharmacology
Regenerative pharmacology
Biologicals and biotherapeutics
Translational pharmacology
Nutriceutical pharmacology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


