Toll-like receptor signaling in neurons modulates C. elegans feeding behavior in a hunger state-dependent manner
IF 8.8
2区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
Animals face the risk of encountering pathogenic microbes while foraging for resources. Assessing the risk of nutrition vs. infection can result in the behavioral regulation of immune processes. Behavioral immunity in the nematode roundworm Caenorhabditis elegans (C. elegans) is regulated, in part, by the innate immune molecule TOL-1: a homolog of vertebrate Toll-like Receptor (TLR) proteins that influences C. elegans pathogen avoidance behaviors by promoting the development of CO2-detecting chemosensory neurons. While TOL-1′s role in pathogen avoidance is well established, its role in an opposing behavior – foraging – has not been examined. In addition to pathogenic bacteria, preferred food for C. elegans, such as Escherichia coli (E. coli), create significant and aversive environmental CO2 levels which may limit feeding behaviors in a tol-1 dependent manner. We have found that in addition to conferring antibacterial immunity, TOL-1 signals in neurons through the p38 MAPK PMK-1 to promote turning behavior and limit foraging when food is abundant and that the anorectic TOL-1/PMK-1 pathway is attenuated during starvation to promote foraging. These data highlight the dynamic role of a conserved innate immune cascade in neurons during both high and low hunger states and identify mechanisms underlying the neuro-immune control of feeding strategies.
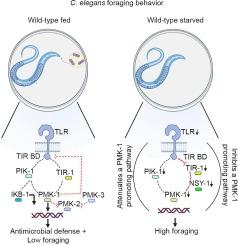
神经元中的Toll样受体信号以饥饿状态依赖的方式调节秀丽隐杆线虫的摄食行为
动物在觅食时面临着遇到病原微生物的风险。评估营养与感染的风险可导致免疫过程的行为调节。线虫蛔虫(Caenorhabditis elegans)的行为免疫部分受先天性免疫分子 TOL-1 的调节:TOL-1 是脊椎动物 Toll 样受体(TLR)蛋白的同源物,它通过促进二氧化碳检测化学感觉神经元的发育来影响线虫的病原体回避行为。虽然 TOL-1 在病原体回避中的作用已得到证实,但它在相反行为--觅食--中的作用却尚未得到研究。除了病原菌外,大肠杆菌(E. coli)等草履虫的首选食物也会产生大量令人厌恶的环境二氧化碳,这可能会以依赖 TOL-1 的方式限制草履虫的觅食行为。我们发现,除了赋予抗菌免疫力外,TOL-1还通过p38 MAPK PMK-1在神经元中发出信号,在食物丰富时促进翻身行为并限制觅食,而在饥饿时,厌食的TOL-1/PMK-1通路会减弱,以促进觅食。这些数据强调了神经元中保守的先天性免疫级联在高饥饿和低饥饿状态下的动态作用,并确定了神经免疫控制觅食策略的机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
29.60
自引率
2.00%
发文量
290
审稿时长
28 days
期刊介绍:
Established in 1987, Brain, Behavior, and Immunity proudly serves as the official journal of the Psychoneuroimmunology Research Society (PNIRS). This pioneering journal is dedicated to publishing peer-reviewed basic, experimental, and clinical studies that explore the intricate interactions among behavioral, neural, endocrine, and immune systems in both humans and animals.
As an international and interdisciplinary platform, Brain, Behavior, and Immunity focuses on original research spanning neuroscience, immunology, integrative physiology, behavioral biology, psychiatry, psychology, and clinical medicine. The journal is inclusive of research conducted at various levels, including molecular, cellular, social, and whole organism perspectives. With a commitment to efficiency, the journal facilitates online submission and review, ensuring timely publication of experimental results. Manuscripts typically undergo peer review and are returned to authors within 30 days of submission. It's worth noting that Brain, Behavior, and Immunity, published eight times a year, does not impose submission fees or page charges, fostering an open and accessible platform for scientific discourse.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


