Bacterial flotillins as destabilizers of phospholipid membranes
IF 2.5
3区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
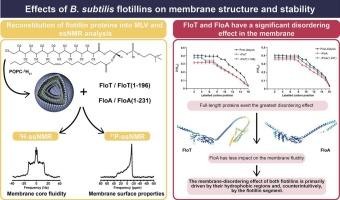
From archaea to mammals evolutionary conserved flotillins are scaffolding proteins, recognized for their nandomain-segregating activity. Flotillins form basket-like oligomeric architectures on the membrane, based on a conserved secondary structure composition of the monomeric subunits: a membrane-targeting region, an SPFH domain and a coiled-coil “flotillin” domain. In B. subtilis, the two flotillins FloT and FloA are present, localizing mainly in distinct nanodomains and executing multiple cellular functions. We here use deuterium and phosphorus solid-state NMR to monitor the effect of the different flotillins FloT and FloA and their structural components on model membranes. We find a clear disordering effect of FloT and FloA on the membranes reaching the carbon positions in the centre of the membrane. This effect is imposed by the hydrophobic region and the adjacent SPFH domain and, surprisingly, further supported by the membrane-distant flotillin domain. Biological implications of this disordering action are discussed.
作为磷脂膜脱稳剂的细菌絮凝物。
从古生菌到哺乳动物,在进化过程中得到保护的绒毛膜蛋白是一种支架蛋白,因其具有核苷酸聚集活性而得到认可。基于单体亚基保守的二级结构组成:一个膜靶区、一个 SPFH 结构域和一个线圈 "flotillin "结构域,磷脂酰蛋白在膜上形成篮状寡聚体结构。在枯草芽孢杆菌中,存在 FloT 和 FloA 两种菌素,它们主要定位于不同的纳米域中,执行多种细胞功能。在此,我们利用氘和磷固态核磁共振来监测不同的FloT和FloA及其结构成分对模型膜的影响。我们发现 FloT 和 FloA 对到达膜中心碳位置的膜有明显的失调效应。这种作用是由疏水区域和邻近的 SPFH 结构域造成的,而且令人惊讶的是,这种作用还得到了膜远端 flotillin 结构域的进一步支持。本文讨论了这种紊乱作用的生物学意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biochimica et biophysica acta. Biomembranes
生物-生化与分子生物学
CiteScore
8.20
自引率
5.90%
发文量
175
审稿时长
2.3 months
期刊介绍:
BBA Biomembranes has its main focus on membrane structure, function and biomolecular organization, membrane proteins, receptors, channels and anchors, fluidity and composition, model membranes and liposomes, membrane surface studies and ligand interactions, transport studies, and membrane dynamics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


