Antimicrobial and antibiofilm potential of α-MSH derived cationic and hydrophobic peptides against Escherichia coli: Mechanistic insight through peptide-lipopolysaccharide interactions
IF 2.5
3区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
The prevalence of infections caused by various Gram-negative pathogens specifically Escherichia coli continuously poses a significant challenge in health care as well as community settings owing to their ability to form biofilm and escalating tolerance towards available antibiotics. While most treatment regimes are targeted at eliminating the E. coli cells, the pathogenicity factors called endotoxin (lipopolysaccharides), associated with the sepsis initiation and the leading cause of death in intensive care units globally, are often ignored. In this study, the potency of alpha-melanocyte stimulating hormone based-peptides, particularly Ana-9 and Ana-10 against E. coli was investigated through microbiological, biophysical, and microscopic assays. Both Ana-9 and Ana-10 demonstrated enhanced activity against planktonic E. coli cells, and retained their activity against biofilm, which was supported by confocal microscopy. From the mechanistic perspective, spectroscopic studies indicated that the binding of peptides with LPS led to structural alteration of peptides due to their insertion into the hydrophobic environment of LPS. The electrostatic interaction of the peptide with LPS leads to outer membrane disorganization, allowing the peptide to access the inner membrane, depolarize it and ultimately inhibit the bacterial cells within the biofilm. These observations were further confirmed by atomic force and scanning electron microscopy. Thus, this study deepens our understanding of the structural characteristics of peptides attached to LPS, which could lead to the gradual improvement in developing more potent, broad-spectrum endotoxin neutralizers.
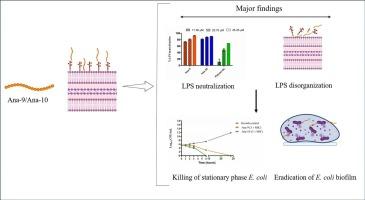
α-MSH衍生的阳离子肽和疏水肽对大肠杆菌的抗菌和抗生物膜潜力:通过肽与脂多糖相互作用的机理研究。
由于各种革兰氏阴性病原体,特别是大肠埃希氏菌,具有形成生物膜的能力,而且对现有抗生素的耐受性不断提高,因此它们引起的感染在医疗保健和社区环境中持续流行,构成了一项重大挑战。虽然大多数治疗方案都以消灭大肠杆菌细胞为目标,但被称为内毒素(脂多糖)的致病因子却常常被忽视,而内毒素与败血症的发生有关,是全球重症监护室的主要死因。在这项研究中,我们通过微生物学、生物物理学和显微镜实验研究了基于α-黑色素细胞刺激素的肽,特别是 Ana-9 和 Ana-10 对大肠杆菌的作用。Ana-9 和 Ana-10 对浮游大肠杆菌细胞的活性增强,对生物膜的活性保持不变,共聚焦显微镜证实了这一点。从机理角度来看,光谱研究表明,肽与 LPS 结合后,由于肽插入到 LPS 的疏水环境中,导致肽的结构发生了改变。多肽与 LPS 的静电作用导致外膜紊乱,使多肽进入内膜,使内膜去极化,最终抑制生物膜内的细菌细胞。原子力和扫描电子显微镜进一步证实了这些观察结果。因此,这项研究加深了我们对附着在 LPS 上的肽的结构特征的了解,这将有助于逐步开发出更强效、更广谱的内毒素中和剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biochimica et biophysica acta. Biomembranes
生物-生化与分子生物学
CiteScore
8.20
自引率
5.90%
发文量
175
审稿时长
2.3 months
期刊介绍:
BBA Biomembranes has its main focus on membrane structure, function and biomolecular organization, membrane proteins, receptors, channels and anchors, fluidity and composition, model membranes and liposomes, membrane surface studies and ligand interactions, transport studies, and membrane dynamics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


