Yohimbine treatment improves pulmonary fibrosis by attenuating the inflammation and oxidative stress via modulating the MAPK pathway
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Idiopathic pulmonary fibrosis (IPF) is a devastating interstitial lung disorder characterized by the accumulation of extracellular matrix and collagen, resulting in significant parenchymal scarring and respiratory failure that leads to mortality. Yohimbine (YBH) is an α-2 adrenergic receptor antagonist with anti-oxidant and anti-inflammatory properties. In the current study, we aimed to investigate the anti-inflammatory, anti-oxidant and anti-fibrotic activity of YBH against LPS/TGF-β-induced differentiation in BEAS-2B/LL29 cells and bleomycin (BLMN) induced pulmonary fibrosis model in rats. Network pharmacology, gene expression, Western-blot analysis, immune-cytochemistry/immunohistochemistry, lung functional analysis, and histology techniques were used to assess the fibrotic marker expression/levels in cells or rat lung tissues. YBH treatment significantly attenuated the LPS-induced pro-inflammatory (identified through a network-pharmacology approach) and oxidative stress markers expression in lung epithelial cells. TGF-β stimulation significantly elevated the fibrotic cascade of markers and treatment with YBH attenuated these markers’ expression/levels. Intra-tracheal administration of BLMN caused a significant elevation of various inflammatory/oxidative stress and fibrotic markers expression in lung tissues and treatment with YBH significantly mitigated the same. Ashcroft score analysis revealed that BLMN exhibited severe distortion of the lungs, elevation of thickness of the alveolar walls and accumulation of collagen in tissues, further treatment with YBH significantly suppressed these events and improved the lung architecture. Lung functional parameters demonstrated that BLMN-induced stiffness and resistance were reduced considerably upon YBH treatment and restored lung function dose-dependently. Overall, this study reveals that YBH treatment significantly attenuated the BLMN-induced fibrosis by regulating the MAPK pathway and provided insightful information for progressing towards translational outcomes.
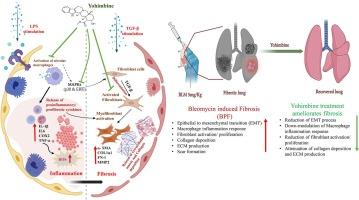
育亨宾通过调节 MAPK 通路减轻炎症和氧化应激,从而改善肺纤维化。
特发性肺纤维化(IPF)是一种破坏性的间质性肺部疾病,其特点是细胞外基质和胶原蛋白堆积,导致肺实质严重瘢痕形成和呼吸衰竭,从而导致死亡。育亨宾(YBH)是一种α-2肾上腺素能受体拮抗剂,具有抗氧化和抗炎特性。本研究旨在探讨育亨宾对LPS/TGF-β诱导的BEAS-2B/LL29细胞分化和博莱霉素(BLMN)诱导的大鼠肺纤维化模型的抗炎、抗氧化和抗纤维化活性。采用网络药理学、基因表达、Western-blot分析、免疫细胞化学/免疫组织化学、肺功能分析和组织学技术来评估细胞或大鼠肺组织中纤维化标志物的表达/水平。YBH治疗可明显减轻LPS诱导的肺上皮细胞促炎(通过网络药理学方法确定)和氧化应激标记物的表达。TGF-β 刺激可显著提高纤维化级联标记物的表达,而 YBH 治疗可减轻这些标记物的表达/水平。气管内给药 BLMN 会导致肺组织中各种炎症/氧化应激和纤维化标志物的表达明显升高,而使用 YBH 治疗则会明显减轻这种情况。Ashcroft 评分分析表明,BLMN 表现出肺部严重变形、肺泡壁厚度增加和组织中胶原蛋白堆积,进一步使用 YBH 治疗可明显抑制这些现象并改善肺部结构。肺功能参数表明,经 YBH 治疗后,BLMN 引起的肺僵化和阻力大大降低,肺功能的恢复与剂量有关。总之,本研究揭示了 YBH 治疗可通过调节 MAPK 通路显著减轻 BLMN 诱导的纤维化,并为取得转化成果提供了有价值的信息。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


