Cladophorol-A is an inhibitor of cyclic GMP-AMP synthase
IF 2.5
4区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Cyclic guanosine monophosphate (GMP)-adenosine monophosphate (AMP) synthase (cGAS) is an enzyme sensor of double-stranded DNA (dsDNA) that serves to trigger activation of the cGAS-stimulator of interferon genes (STING) pathway. Excessive activation of this pathway has been demonstrated to contribute to various forms of inflammatory disease. As such, cGAS has arisen as a potential therapeutic target with broad potential applications. Using a pathway-targeted cell-based screening approach, we identified the natural product Cladophorol-A as a new class of non-cytotoxic cGAS inhibitor (cell-based IC50 = 370 nM). An X-ray co-crystal structure at 2.75 Å resolution revealed that Cladophorol-A inhibits cGAS by binding to its active site within the conserved adenosine nucleobase binding site.
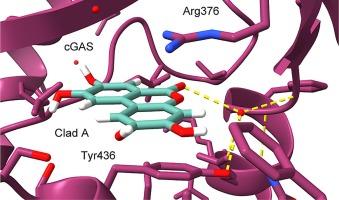
Cladophorol-A 是一种环 GMP-AMP 合成酶抑制剂。
环鸟苷单磷酸(GMP)-腺苷单磷酸(AMP)合成酶(cGAS)是双链 DNA(dsDNA)的一种酶传感器,可触发 cGAS-干扰素基因刺激器(STING)通路的激活。该通路的过度激活已被证实会导致各种形式的炎症性疾病。因此,cGAS 已成为一个潜在的治疗靶点,具有广泛的应用前景。通过基于细胞的通路靶向筛选方法,我们发现天然产物 Cladophorol A 是一种新型的非细胞毒性 cGAS 抑制剂(基于细胞的 IC50 = 370 nM)。分辨率为 2.75 Å 的 X 射线共晶体结构显示,Cladophorol A 可通过与保守的腺苷核碱基结合位点内的活性位点结合来抑制 cGAS。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.70
自引率
3.70%
发文量
463
审稿时长
27 days
期刊介绍:
Bioorganic & Medicinal Chemistry Letters presents preliminary experimental or theoretical research results of outstanding significance and timeliness on all aspects of science at the interface of chemistry and biology and on major advances in drug design and development. The journal publishes articles in the form of communications reporting experimental or theoretical results of special interest, and strives to provide maximum dissemination to a large, international audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


