Assembly of the Human Multi-tRNA Synthetase Complex Through Leucine Zipper Motifs
IF 4.7
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
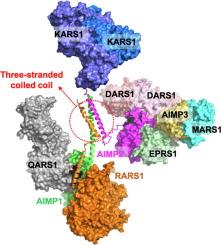
Aminoacyl-tRNA synthetases (ARSs) are responsible for the ligation of amino acids to their cognate tRNAs. In human, nine ARSs form a multi-tRNA synthetase complex (MSC) with three ARS-interacting multifunctional proteins (AIMPs). Among the components of MSC, arginyl-tRNA synthetase 1 (RARS1) and two AIMPs (AIMP1 and AIMP2) have leucine zipper (LZ) motifs, which they utilize for their assembly in an MSC. RARS1 and AIMP1 have two LZ motifs (LZ1 and LZ2) in their N-terminus, respectively, while AIMP2 has one LZ motif between its lysyl-tRNA synthetase 1 (KARS1)-binding motif and glutathione transferase-homology domain, which links aspartyl-tRNA synthetase 1 (DARS1). Although the interaction mode between AIMP1 and RARS1, which also binds glutaminyl-tRNA synthetase 1 (QARS1), has been revealed, the mode in the presence of AIMP2 is still ambiguous since AIMP2 is known to not only bind to AIMP1 but also form a homodimer through its LZ. Here, we determined a crystal structure of the LZ complex of AIMP1 and AIMP2 and revealed the interaction mode of a heterotrimeric complex of RARS1, AIMP1, and AIMP2. The complex is established by a three-stranded coiled-coil structure with RARS1 LZ1, AIMP1 LZ1, and AIMP2 LZ and is completed with a two-stranded coiled-coil structure of RARS1 LZ2 and AIMP1 LZ2. In the human MSC, this heterotrimeric complex of RARS1, AIMP1, and AIMP2 allows for a subcomplex of fourteen protein molecules, in which two QARS1-RARS1-AIMP1-AIMP2-2 × KARS1 complexes are linked separately to a dimeric DARS1.
通过亮氨酸拉链图案组装人类多 tRNA 合成酶复合物。
氨基酰-tRNA 合成酶(ARSs)负责将氨基酸连接到它们的同源 tRNA 上。在人类体内,九个 ARS 与三个 ARS 相互作用的多功能蛋白(AIMPs)组成了一个多 tRNA 合成酶复合物(MSC)。在 MSC 的组成成分中,精氨酰-tRNA 合成酶 1(RARS1)和两个 AIMPs(AIMP1 和 AIMP2)具有亮氨酸拉链(LZ)基序,它们利用这些基序组装成 MSC。RARS1 和 AIMP1 的 N 端分别有两个 LZ 基序(LZ1 和 LZ2),而 AIMP2 的赖氨酰-tRNA 合成酶 1(KARS1)结合基序和谷胱甘肽转移酶同源结构域之间有一个 LZ 基序,该结构域连接天冬氨酰-tRNA 合成酶 1(DARS1)。虽然 AIMP1 与 RARS1(RARS1 也能结合谷氨酰胺酰-tRNA 合成酶 1(QARS1))之间的相互作用模式已被揭示,但由于已知 AIMP2 不仅能与 AIMP1 结合,还能通过其 LZ 形成同源二聚体,因此在 AIMP2 存在时的相互作用模式仍不明确。在这里,我们测定了 AIMP1 和 AIMP2 的 LZ 复合物的晶体结构,并揭示了 RARS1、AIMP1 和 AIMP2 的异源三聚体复合物的相互作用模式。该复合物由 RARS1 LZ1、AIMP1 LZ1 和 AIMP2 LZ 的三股线圈结构构成,并由 RARS1 LZ2 和 AIMP1 LZ2 的两股线圈结构完成。在人类间充质干细胞中,这种由 RARS1、AIMP1 和 AIMP2 组成的异三聚体复合物可形成由 14 个蛋白质分子组成的亚复合物,其中两个 QARS1-RARS1-AIMP1-AIMP2-2×KARS1 复合物分别与二聚体 DARS1 相连。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Biology
生物-生化与分子生物学
CiteScore
11.30
自引率
1.80%
发文量
412
审稿时长
28 days
期刊介绍:
Journal of Molecular Biology (JMB) provides high quality, comprehensive and broad coverage in all areas of molecular biology. The journal publishes original scientific research papers that provide mechanistic and functional insights and report a significant advance to the field. The journal encourages the submission of multidisciplinary studies that use complementary experimental and computational approaches to address challenging biological questions.
Research areas include but are not limited to: Biomolecular interactions, signaling networks, systems biology; Cell cycle, cell growth, cell differentiation; Cell death, autophagy; Cell signaling and regulation; Chemical biology; Computational biology, in combination with experimental studies; DNA replication, repair, and recombination; Development, regenerative biology, mechanistic and functional studies of stem cells; Epigenetics, chromatin structure and function; Gene expression; Membrane processes, cell surface proteins and cell-cell interactions; Methodological advances, both experimental and theoretical, including databases; Microbiology, virology, and interactions with the host or environment; Microbiota mechanistic and functional studies; Nuclear organization; Post-translational modifications, proteomics; Processing and function of biologically important macromolecules and complexes; Molecular basis of disease; RNA processing, structure and functions of non-coding RNAs, transcription; Sorting, spatiotemporal organization, trafficking; Structural biology; Synthetic biology; Translation, protein folding, chaperones, protein degradation and quality control.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


