Fucoidan-derived carbon dots as nanopenetrants of blood-brain barrier for Parkinson’s disease treatment
IF 9.7
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Parkinson’s Disease (PD) stands as a prevalent neurodegenerative disorder. However, current pharmacotherapies for PD face challenges due to inadequate penetration through the blood–brain barrier (BBB), posing limitations on their therapeutic efficacy. Considering the potential of negatively charged carbon dots (CDs) in retaining functional groups from precursor molecules and vertically crossing the BBB, this study focuses on the utilization of fucoidan (FD), a promising pharmaceutical candidate with neuroprotective effects on dopamine-active neurons, for the development of negatively charged CDs through a one-step hydrothermal method, aiming to achieve efficient BBB penetration for PD treatment. The obtained fucoidan-derived carbon dots (FDCDs) exhibit the fundamental characteristics of CDs, such as nanostructure particles with an average diameter of less than 10 nm and significant photoluminescence ability. They also retain the abundant functional groups of SO42- from FD, resulting in a negatively charged surface. In vitro cell experiments were conducted to validate the ability of FDCDs to mitigate 1-Methyl-4-phenylpyridinium ion (MPP+)-induced damage in PC12 cells via anti-inflammatory pathway, antioxidant capacity, and anti-apoptotic effect. After confirming the ability of FDCDs to traverse the BBB using 3D small animal imaging, the intravenous administration of FDCDs via tail injection was observed to successfully restore the motor function in 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD mice. Notably, no apparent biotoxic response was observed, highlighting the promising potential of FDCDs for effective PD therapy.
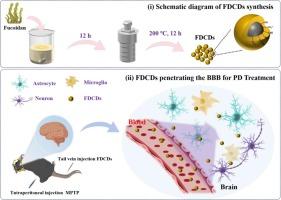
褐藻糖胶衍生碳点作为用于帕金森病治疗的血脑屏障纳米渗透剂。
帕金森病(PD)是一种常见的神经退行性疾病。然而,目前治疗帕金森病的药物疗法由于无法充分穿透血脑屏障(BBB)而面临挑战,从而限制了其疗效。考虑到带负电荷的碳点(CD)在保留前体分子中的功能基团并垂直穿过血脑屏障方面的潜力,本研究重点利用褐藻糖胶(FD)这种对多巴胺活性神经元具有神经保护作用的前景看好的候选药物,通过一步水热法开发带负电荷的碳点,旨在实现高效的血脑屏障穿透,从而治疗帕金森病。所获得的褐藻糖胶衍生碳点(FDCDs)具有 CD 的基本特征,如平均直径小于 10 纳米的纳米结构颗粒和显著的光致发光能力。它们还保留了 FD 中 SO42- 的丰富官能团,从而形成了带负电荷的表面。体外细胞实验验证了 FDCDs 通过抗炎途径、抗氧化能力和抗凋亡作用减轻 1-甲基-4-苯基吡啶鎓离子(MPP+)诱导的 PC12 细胞损伤的能力。利用三维小动物成像技术确认了 FDCDs 穿过 BBB 的能力后,通过尾部注射静脉注射 FDCDs 成功地恢复了 1-甲基-4-苯基-1,2,3,6-四氢吡啶(MPTP)诱导的帕金森病小鼠的运动功能。值得注意的是,没有观察到明显的生物毒性反应,这凸显了 FDCDs 在有效治疗帕金森病方面的巨大潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
16.10
自引率
7.10%
发文量
2568
审稿时长
2 months
期刊介绍:
The Journal of Colloid and Interface Science publishes original research findings on the fundamental principles of colloid and interface science, as well as innovative applications in various fields. The criteria for publication include impact, quality, novelty, and originality.
Emphasis:
The journal emphasizes fundamental scientific innovation within the following categories:
A.Colloidal Materials and Nanomaterials
B.Soft Colloidal and Self-Assembly Systems
C.Adsorption, Catalysis, and Electrochemistry
D.Interfacial Processes, Capillarity, and Wetting
E.Biomaterials and Nanomedicine
F.Energy Conversion and Storage, and Environmental Technologies

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


