PRDX6 contributes to selenocysteine metabolism and ferroptosis resistance
IF 14.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Selenocysteine (Sec) metabolism is crucial for cellular function and ferroptosis prevention and begins with the uptake of the Sec carrier, selenoprotein P (SELENOP). Following uptake, Sec released from SELENOP is metabolized via selenocysteine lyase (SCLY), producing selenide, a substrate for selenophosphate synthetase 2 (SEPHS2), which provides the essential selenium donor, selenophosphate (H2SePO3−), for the biosynthesis of the Sec-tRNA. Here, we discovered an alternative pathway in Sec metabolism mediated by peroxiredoxin 6 (PRDX6), independent of SCLY. Mechanistically, we demonstrate that PRDX6 can readily react with selenide and interact with SEPHS2, potentially acting as a selenium delivery system. Moreover, we demonstrate the functional significance of this alternative route in human cancer cells, revealing a notable association between elevated expression of PRDX6 and human MYCN-amplified neuroblastoma subtype. Our study sheds light on a previously unrecognized aspect of Sec metabolism and its implications in ferroptosis, offering further possibilities for therapeutic exploitation.
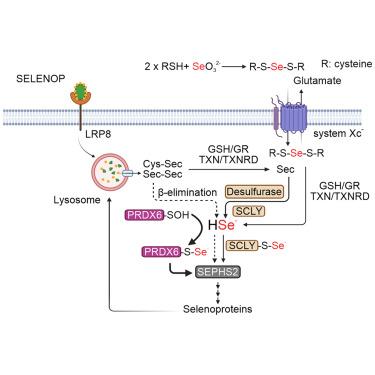
PRDX6 促进硒代半胱氨酸代谢和抗铁病能力
硒代半胱氨酸(Sec)代谢对细胞功能和预防铁变态反应至关重要,它始于硒代半胱氨酸载体硒蛋白 P(SELENOP)的吸收。摄取后,从 SELENOP 释放的 Sec 通过硒半胱氨酸裂解酶(SCLY)进行代谢,产生硒化物,硒化物是硒磷酸盐合成酶 2(SEPHS2)的底物,后者为 Sec-tRNA 的生物合成提供必要的硒供体硒磷酸盐(H2SePO3-)。在这里,我们发现了由过氧化物歧化酶 6(PRDX6)介导的独立于 SCLY 的 Sec 代谢的另一条途径。从机理上讲,我们证明了 PRDX6 很容易与硒化物发生反应,并与 SEPHS2 相互作用,从而可能充当硒传递系统。此外,我们还证明了这一替代途径在人类癌细胞中的功能意义,揭示了 PRDX6 表达升高与人类 MYCN 扩增神经母细胞瘤亚型之间的显著关联。我们的研究揭示了 Sec 代谢中以前未被认识到的一个方面及其在铁跃迁中的影响,为治疗提供了更多可能性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Cell
生物-生化与分子生物学
CiteScore
26.00
自引率
3.80%
发文量
389
审稿时长
1 months
期刊介绍:
Molecular Cell is a companion to Cell, the leading journal of biology and the highest-impact journal in the world. Launched in December 1997 and published monthly. Molecular Cell is dedicated to publishing cutting-edge research in molecular biology, focusing on fundamental cellular processes. The journal encompasses a wide range of topics, including DNA replication, recombination, and repair; Chromatin biology and genome organization; Transcription; RNA processing and decay; Non-coding RNA function; Translation; Protein folding, modification, and quality control; Signal transduction pathways; Cell cycle and checkpoints; Cell death; Autophagy; Metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


