Construction of Axially Chiral 4-Aminoquinolines by Cycloaddition and Central-to-Axial Chirality Conversion
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A two-step strategy has been established for the enantioselective synthesis of 4-aminoquinolines possessing axial chirality. This approach involves a chiral phosphoric acid-catalyzed cycloaddition, followed by a DDQ oxidation step. The method offers efficient access to a variety of 1,1′-biaryl-2,2′-amino alcohol derivatives in excellent yields and enantioselectivities (up to 98% yield and 93% ee). Furthermore, the synthetic transformation of the products was also investigated.
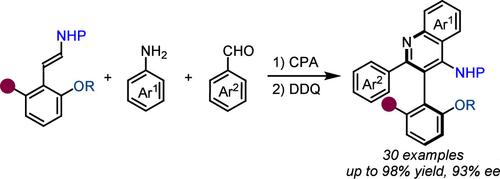
通过环加成和中心手性到轴手性的转换构建轴手性 4-氨基喹啉类化合物
我们建立了一种两步法,用于对映选择性合成具有轴向手性的 4-氨基喹啉。该方法包括手性磷酸催化的环加成,然后是 DDQ 氧化步骤。该方法可以高效地获得多种 1,1′-联-2,2′-氨基醇衍生物,而且产率和对映选择性极佳(产率高达 98%,ee 值高达 93%)。此外,还研究了这些产品的合成转化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


