Rhodium-Catalyzed Asymmetric Hydrogenation and Transfer Hydrogenation of α-Nitro Ketones
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A catalytic protocol for the enantioselective hydrogenation and transfer hydrogenation of α-nitro ketones was developed, providing a wide range of β-nitro-α-phenylethanols with high yields and excellent enantioselectivities (up to 98% yield and up to >99.9% ee). Compatibility with a wide range of solvents and bases demonstrates the robustness of this reaction. The synthetic potential of the protocol was demonstrated by the high TON experiment as well as the application in the synthesis of key intermediates of mirabegron (S/C = 10,000, 95% yield, 99% ee).
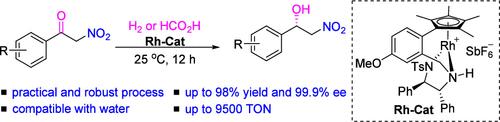
铑催化的 α-硝基酮的不对称氢化和转移氢化反应
研究人员开发了一种用于α-硝基酮对映体选择性氢化和转移氢化的催化方案,该方案提供了多种β-硝基-α-苯乙醇,具有高产率和优异的对映体选择性(产率高达 98%,ee高达 99.9%)。与多种溶剂和碱的兼容性表明了该反应的稳健性。高吨位实验以及米拉贝琼关键中间体(S/C = 10,000,收率 95%,ee 99%)的合成应用证明了该方案的合成潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


