In Situ ATR-IR Spectroscopy for the Degradation of Acetaminophen on WO3–AgCl Photocatalysts
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Photocatalysts made of WO3 coupled with AgCl have demonstrated enhanced photocatalytic activity for degrading organic contaminants. While various studies have focused on the degradation of organic molecules and the formation of intermediate species during photocatalysis, the catalytic interface during illumination has received less attention. Attenuated total reflection infrared spectroscopy (ATR-IR) is ideal for studying solid–liquid interfaces and has shown potential for studying catalytic reactions. In this study, the application of in situ ATR-IR for the degradation of the model contaminant acetaminophen using pure WO3 and WO3–AgCl with and without a surface-directing agent (citric acid) was explored. ATR-IR experiments in batch and continuous flow modes were conducted to characterize the interface and its adsorbates during illumination. Liquid chromatography–high-resolution mass spectrometry (LC-HRMS) was used to further explore the reaction intermediates and to support the ATR-IR data. ACT dimers, ACT trimers, dimers of ACT and p-benzoquinone, and p-benzoquinone were identified as degradation products. NH3-TPD was employed to investigate the surface acidity and its role in the photocatalytic process, revealing that the combination of AgCl with WO3 reduced the number of weak and moderate acidic sites, while the addition of citric acid promoted the formation of strong acidic sites in the photocatalyst, which significantly impacted the degradation efficiency. Some limitations of the ATR-IR technique were highlighted; nevertheless, it remains a powerful tool for probing solid–liquid interfaces during photocatalytic degradation.
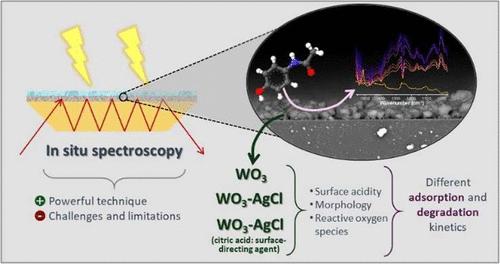
在 WO3-AgCl 光催化剂上降解对乙酰氨基酚的原位 ATR-IR 光谱法
由 WO3 和 AgCl 制成的光催化剂在降解有机污染物方面具有更强的光催化活性。虽然各种研究都关注光催化过程中有机分子的降解和中间产物的形成,但对光照过程中的催化界面关注较少。衰减全反射红外光谱(ATR-IR)是研究固液界面的理想方法,在研究催化反应方面已显示出潜力。本研究探索了原位 ATR-IR 在使用纯 WO3 和 WO3-AgCl(含或不含表面引导剂(柠檬酸))降解模式污染物对乙酰氨基酚中的应用。在间歇和连续流模式下进行了 ATR-IR 实验,以确定照明过程中界面及其吸附物的特征。液相色谱-高分辨质谱法(LC-HRMS)用于进一步探索反应中间产物,并为 ATR-IR 数据提供支持。ACT 二聚体、ACT 三聚体、ACT 和对苯醌的二聚体以及对苯醌被鉴定为降解产物。采用 NH3-TPD 研究了表面酸度及其在光催化过程中的作用,结果表明,AgCl 与 WO3 的结合减少了弱酸性和中等酸性位点的数量,而柠檬酸的加入则促进了光催化剂中强酸性位点的形成,从而显著影响了降解效率。ATR-IR 技术的一些局限性得到了强调;然而,它仍然是光催化降解过程中探测固液界面的有力工具。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


