Nitrogenation of Alkynes with Nitrones to Prepare Functionalized [1,4]Oxazinones through Csp–Csp2 Bond Cleavage
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Herein, we report a novel strategy of hypervalent iodine(III) compound-mediated selective Csp–Csp2 bond cleavage of alkynes and C═N/N–O bond cleavage of nitrones and recombination of C–C/C–O/C–N multiple bonds to access various functionalized [1,4]oxazinones bearing a vicinal carbon stereocenter in good yields and high diastereoselectivity. Mechanistic studies revealed that the reaction undergoes a domino [4 + 3] cycloaddition, 1,3-rearrangement of N–O bond, intramolecular cyclization, dearomatization, and rearomatization over four steps in a single flask. The present method features good functional group tolerance, broad substrate scope, and C–C/C═N/N–O multiple bonds cleavage and recombination.
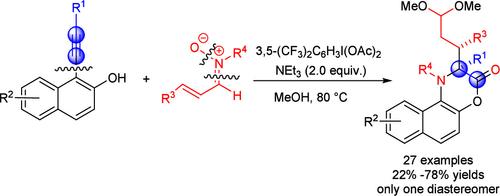
通过 Csp-Csp2 键裂解将炔烃与硝基化合物氮化以制备功能化 [1,4]Oxazinones
在此,我们报告了一种新策略,即通过超价碘(III)化合物介导的炔类化合物选择性 Csp-Csp2 键裂解和硝基化合物的 C═N/N-O 键裂解以及 C-C/C-O/C-N 多键重组,以良好的产率和高非对映选择性获得各种带有邻碳立体中心的官能化 [1,4]oxazinones。机理研究表明,该反应在一个烧瓶中经历了多米诺[4 + 3]环加成、N-O 键的 1,3 重排、分子内环化、脱芳基化和重芳基化四个步骤。该方法具有良好的官能团耐受性、广泛的底物范围、C-C/C═N/N-O 多键裂解和重组等特点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


