Role of the PGAM5-CypD mitochondrial pathway in methylglyoxal-induced bone loss in diabetic osteoporosis
IF 3.5
2区 医学
Q2 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
Diabetic osteoporosis (DOP) is a skeletal complication with a high rate of disability. It results in a great burden to the patient's family and society. Methylglyoxal (MG) is a toxic by-product of the glycolytic process that occurs during diabetic conditions. It causes osteoblastic injury and con-tributes to the initiation and development of DOP. Disruption of mitochondrial homeostasis has been implicated as a cause of dysregulated osteo-blastogenesis, an essential step in bone formation. It is unclear whether mitochondrial dysfunction is involved in MG-induced osteoblast dysfunction. In this study, we showed that mitochondrial dysfunction contributes to MG-induced MC3T3-E1 cell apoptosis and impaired differentiation. A significant reduction of mitochondrial membrane potential (MMP) and ATP production occurred in MG-induced osteoblasts as well as increasing mitochondrial reactive oxygen species (mtROS) and intracellular Ca2+. Classical antioxidant N-Acetylcysteine (NAC) significantly attenuated mitochondrial dysfunction as well as osteoblast apoptosis and osteogenic differentiation damage induced by MG. More importantly, we found that activating phosphoglycerate mutase family member 5 (PGAM5) and cyclophilin D (CypD), which contributes to mitochondrial homeostasis, is involved in MG-induced osteoblast injury. Both PGAM5 and CypD knockdown effectively reversed osteoblast viability and function, whereas PGAM5 or CypD overexpression aggravated osteoblast injury caused by MG. Moreover, the result of co-transfection revealed that PGAM5 is an upstream signaling molecule of CypD. By constructing type I diabetes mouse models, we further found that the expression of PGAM5 and CypD were both increased in the femur along with a reduction of ATP and increased TUNEL-positive cells. These results, for the first time, suggest that MG-induced mitochondrial dysfunction induces osteoblast injury through the PGAM5-CypD pathway. This study provides insight into the prevention and treatment of DOP.
Lay summary
This study highlights the role of mitochondria in regulating osteoblast viability and function under conditions of diabetic osteoporosis (DOP). We found that the PGAM5-CypD mitochondrial pathway is activated following glycolytic by-product methylglyoxal (MG) treatment, which contributes to mitochondrial dysfunction and osteogenic dysfunction. This mechanism implicates mitochondria as a potential therapeutic target for osteoporosis.
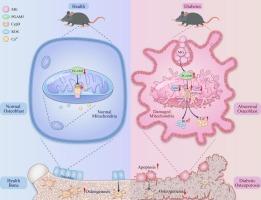
PGAM5-CypD 线粒体途径在甲基乙二醛诱导的糖尿病骨质疏松症骨质流失中的作用。
糖尿病骨质疏松症(DOP)是一种骨骼并发症,致残率很高。它给患者家庭和社会造成了巨大负担。甲基乙二醛(MG)是糖尿病患者糖酵解过程中产生的一种有毒副产物。它会导致成骨细胞损伤,并导致 DOP 的发生和发展。线粒体平衡的破坏被认为是骨细胞生成失调的一个原因,而骨细胞生成是骨形成的一个重要步骤。目前尚不清楚线粒体功能障碍是否与 MG 诱导的成骨细胞功能障碍有关。在这项研究中,我们发现线粒体功能障碍导致了 MG 诱导的 MC3T3-E1 细胞凋亡和分化受损。在 MG 诱导的成骨细胞中,线粒体膜电位(MMP)和 ATP 生成明显降低,线粒体活性氧(mtROS)和细胞内 Ca2+ 增加。经典抗氧化剂 N-乙酰半胱氨酸(NAC)能显著减轻线粒体功能障碍以及 MG 诱导的成骨细胞凋亡和成骨分化损伤。更重要的是,我们发现活化磷酸甘油酸突变酶家族成员 5(PGAM5)和环嗜酸蛋白 D(CypD)参与了 MG 诱导的成骨细胞损伤。PGAM5和CypD的敲除能有效逆转成骨细胞的活力和功能,而PGAM5或CypD的过表达则会加重MG引起的成骨细胞损伤。此外,联合转染的结果显示,PGAM5是CypD的上游信号分子。通过构建 I 型糖尿病小鼠模型,我们进一步发现 PGAM5 和 CypD 在股骨中的表达均增加,同时 ATP 减少,TUNEL 阳性细胞增加。这些结果首次表明,MG 诱导的线粒体功能障碍通过 PGAM5-CypD 途径诱导成骨细胞损伤。这项研究为预防和治疗 DOP 提供了启示。LAY总结:这项研究强调了线粒体在糖尿病骨质疏松症(DOP)条件下调节成骨细胞活力和功能的作用。我们发现,糖酵解副产物甲基乙二醛(MG)处理后,PGAM5-CypD 线粒体通路被激活,从而导致线粒体功能障碍和成骨功能障碍。这一机制使线粒体成为骨质疏松症的潜在治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bone
医学-内分泌学与代谢
CiteScore
8.90
自引率
4.90%
发文量
264
审稿时长
30 days
期刊介绍:
BONE is an interdisciplinary forum for the rapid publication of original articles and reviews on basic, translational, and clinical aspects of bone and mineral metabolism. The Journal also encourages submissions related to interactions of bone with other organ systems, including cartilage, endocrine, muscle, fat, neural, vascular, gastrointestinal, hematopoietic, and immune systems. Particular attention is placed on the application of experimental studies to clinical practice.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


