Revealing Missing Protein–Ligand Interactions Using AlphaFold Predictions
IF 4.5
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Protein–ligand interactions represent an essential step to understand the bases of molecular recognition, an intense field of research in many scientific areas. Structural biology has played a central role in unveiling protein–ligand interactions, but current techniques are still not able to reliably describe the interactions of ligands with highly flexible regions. In this work, we explored the capacity of AlphaFold2 (AF2) to estimate the presence of interactions between ligands and residues belonging to disordered regions. As these interactions are missing in the crystallographic-derived structures, we called them “ghost interactions”. Using a set of protein structures experimentally obtained after AF2 was trained, we found that the obtained models are good predictors of regions associated with order–disorder transitions. Additionally, we found that AF2 predicts residues making ghost interactions with ligands, which are mostly buried and show differential evolutionary conservation with the rest of the residues located in the flexible region. Our findings could fuel current areas of research that consider, given their biological relevance and their involvement in diseases, intrinsically disordered proteins as potentially valuable targets for drug development.
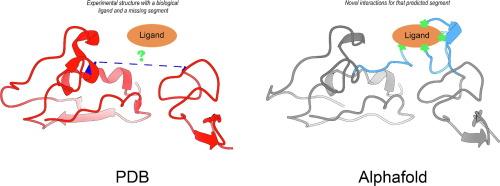
利用 AlphaFold 预测揭示缺失的蛋白质配体相互作用。
蛋白质与配体的相互作用是了解分子识别基础的重要一步,也是许多科学领域的热门研究领域。结构生物学在揭示蛋白质-配体相互作用方面发挥了核心作用,但目前的技术仍无法可靠地描述配体与高柔性区域的相互作用。在这项工作中,我们探索了 AlphaFold2(AF2)估计配体与属于无序区域的残基之间是否存在相互作用的能力。由于这些相互作用在晶体学结构中缺失,我们称之为 "幽灵相互作用"。通过使用一组经过 AF2 训练后通过实验获得的蛋白质结构,我们发现所获得的模型可以很好地预测与有序-无序转换相关的区域。此外,我们还发现 AF2 可以预测与配体发生鬼影作用的残基,这些残基大多被埋藏,并与位于柔性区域的其他残基显示出不同的进化守恒性。鉴于内在无序蛋白质的生物学相关性及其与疾病的关系,我们的研究结果可以推动当前的研究领域,使其成为有价值的潜在药物开发目标。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Biology
生物-生化与分子生物学
CiteScore
11.30
自引率
1.80%
发文量
412
审稿时长
28 days
期刊介绍:
Journal of Molecular Biology (JMB) provides high quality, comprehensive and broad coverage in all areas of molecular biology. The journal publishes original scientific research papers that provide mechanistic and functional insights and report a significant advance to the field. The journal encourages the submission of multidisciplinary studies that use complementary experimental and computational approaches to address challenging biological questions.
Research areas include but are not limited to: Biomolecular interactions, signaling networks, systems biology; Cell cycle, cell growth, cell differentiation; Cell death, autophagy; Cell signaling and regulation; Chemical biology; Computational biology, in combination with experimental studies; DNA replication, repair, and recombination; Development, regenerative biology, mechanistic and functional studies of stem cells; Epigenetics, chromatin structure and function; Gene expression; Membrane processes, cell surface proteins and cell-cell interactions; Methodological advances, both experimental and theoretical, including databases; Microbiology, virology, and interactions with the host or environment; Microbiota mechanistic and functional studies; Nuclear organization; Post-translational modifications, proteomics; Processing and function of biologically important macromolecules and complexes; Molecular basis of disease; RNA processing, structure and functions of non-coding RNAs, transcription; Sorting, spatiotemporal organization, trafficking; Structural biology; Synthetic biology; Translation, protein folding, chaperones, protein degradation and quality control.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


