Iron-Based Layered Perovskite Oxyfluoride Electrocatalyst for Oxygen Evolution: Insights from Crystal Facets with Heteroanionic Coordination
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Mixed-anion compounds have recently attracted attention as solid-state materials that exhibit properties unattainable with those of their single-anion counterparts. However, the use of mixed-anion compounds to control the morphology and engineer the crystal facets of electrocatalysts has been limited because their synthesis method is still immature. This study explored the electrocatalytic properties of a Pb–Fe oxyfluoride, Pb3Fe2O5F2, with a layered perovskite structure for oxygen evolution reaction (OER) and compared its properties in detail with those of a bulk-type cubic three-dimensional (3D) perovskite, PbFeO2F. A Pb3Fe2O5F2 electrode prepared with carbon nanotubes and a graphite sheet as a conductive support and a substrate, respectively, demonstrated better OER performance than a PbFeO2F electrode. The role of specific crystal facets of Pb3Fe2O5F2 in enhancing the OER activity was elucidated through electrochemical analysis. Density functional theory calculations indicated that the Pb3Fe2O5F2 (060) facet with Fe sites exhibited a lower theoretical overpotential for the OER, which was attributed to a moderately strong interaction between the active sites and the reaction intermediates; this interaction was reinforced by the strong electron-withdrawing behavior of fluoride ions. This finding offers new insights for developing efficient electrocatalysts based on oxyfluorides, leveraging the high electronegativity of fluorine to optimize the electronic states at active sites for the OER, without relying on precious metals.
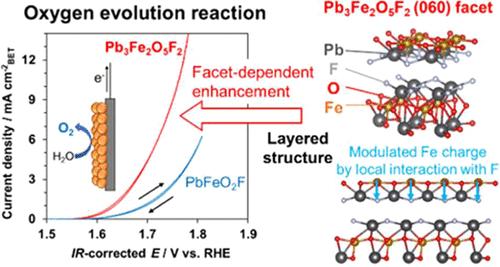
用于氧进化的铁基层状过氧化物氧氟化物电催化剂:异阴离子配位晶面的启示
作为固态材料,混合阴离子化合物最近引起了人们的关注,因为它们具有单阴离子化合物无法达到的特性。然而,由于混合阴离子化合物的合成方法尚不成熟,利用混合阴离子化合物来控制电催化剂的形态和设计其晶面一直受到限制。本研究探索了具有层状包晶结构的氟化铅-铁氧体 Pb3Fe2O5F2 在氧进化反应(OER)中的电催化特性,并将其特性与块状立方三维(3D)包晶 PbFeO2F 进行了详细比较。用碳纳米管和石墨片分别作为导电支架和基底制备的 Pb3Fe2O5F2 电极比 PbFeO2F 电极具有更好的 OER 性能。通过电化学分析,阐明了 Pb3Fe2O5F2 的特定晶面在提高 OER 活性中的作用。密度泛函理论计算表明,具有铁位点的 Pb3Fe2O5F2 (060) 晶面的 OER 理论过电位较低,这归因于活性位点与反应中间产物之间具有中等强度的相互作用;氟离子的强电子抽离行为加强了这种相互作用。这一发现为开发基于氧氟化物的高效电催化剂提供了新的思路,即利用氟的高电负性来优化 OER 活性位点的电子状态,而无需依赖贵金属。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


