An Air-Stable, Single-Component Iridium Precatalyst for the Borylation of C–H Bonds on Large to Miniaturized Scales
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
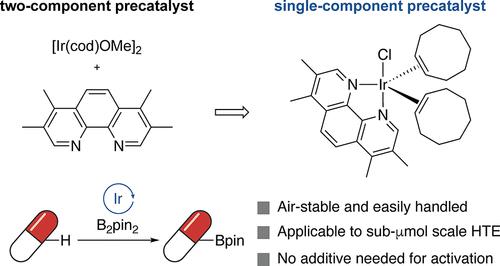
The functionalization of C–H bonds enables the modification of complex molecules, often with the intention of forming compound libraries. The borylation of aryl C–H bonds is a widely used class of C–H bond functionalization, and conventional catalyst systems for the borylation of C–H bonds consist of an iridium source and an N,N-ligand, in conjunction with pinacolborane, to form the active iridium(III) tris(boryl) catalyst. These multicomponent catalyst systems complicate borylation reactions at large and small scales, due to the air sensitivity of the most common iridium precursor [Ir(cod)OMe]2, and, particularly on small scale, the challenges associated with dispensing multiple components with differing solubilities or that are air-sensitive. We describe the discovery of an air-stable, single-component iridium precatalyst, [(tmphen)Ir(coe)2Cl], that generates the same active iridium(III) tris(boryl) catalyst and reacts with higher turnovers, comparable selectivity, and similar scope to those of known catalyst systems for the borylation of aryl and heteroaryl C–H bonds. We show how the development of this precatalyst enables reactions to be run on submicromole scale in a high-throughput experimentation format in conjunction with ChemBead technology, and with a second diversification step that illustrates the potential to diversify structures by chemical sequences involving catalytic reactions, including C–H bond functionalizations, on submicromole scales in the same reaction vessel.
一种空气稳定的单组分铱前催化剂,用于大规模到微型化 C-H 键的硼化反应
通过对 C-H 键进行官能化,可以对复杂的分子进行改性,通常是为了形成化合物库。芳基 C-H 键的硼酸化是一种广泛应用的 C-H 键官能化,传统的 C-H 键硼酸化催化剂体系由铱源和 N,N-配体与频哪醇硼烷组成,形成活性铱(III)三(硼酸)催化剂。由于最常见的铱前驱体[Ir(cod)OMe]2 对空气敏感,特别是在小规模应用中,配制溶解度不同或对空气敏感的多种组分所带来的挑战,这些多组分催化剂体系使大小规模的硼化反应变得复杂。我们介绍了一种空气稳定的单组分铱前催化剂[(tmphen)Ir(coe)2Cl]的发现,它能生成相同活性的铱(III)三(硼)催化剂,并且在芳基和杂芳基 C-H 键的硼化反应中,与已知催化剂体系相比,具有更高的转化率、相似的选择性和类似的反应范围。我们展示了这种前催化剂的开发如何结合 ChemBead 技术,以高通量实验的形式在亚微米尺度上进行反应,以及如何通过涉及催化反应(包括 C-H 键官能化)的化学序列,在同一反应容器中以亚微米尺度进行第二个多样化步骤,从而展示结构多样化的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


