Ten-Step Total Synthesis of (±)-Phaeocaulisin A Enabled by Cyclopropanol Ring-Opening Carbonylation
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
We report an efficient total synthesis of (±)-phaeocaulisin A, a guaianolide sesquiterpene natural product possessing a complex tetracyclic skeleton embedded with an oxaspirolactone and a fused bicyclic lactone, four oxygen-containing stereocenters, and an 8-oxabicyclo[3.2.1]octane core. Our synthesis features a novel palladium-catalyzed cyclopropanol ring-opening carbonylation to access a key γ-ketoester, a chemo- and stereoselective aldol cyclization to form the seven-membered carbocycle, and a cascade ketalization–lactonization to construct the desired tetracyclic skeleton. With these strategically important C–C and C–O bond formation transformations, a 10-step total synthesis of (±)-phaeocaulisin A was achieved. We further developed the cyclopropanol ring-opening carbonylation chemistry to provide an alternative approach to prepare γ-ketoesters. Biologically, the penultimate intermediate with an α-methylene γ-butyrolactone moiety was identified as a promising lead compound with anticancer proliferation activity against a panel of triple-negative or HER2+ breast cancer cell lines.
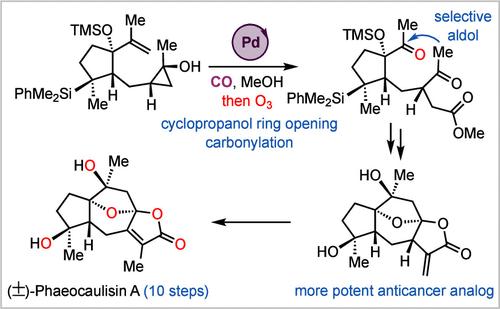
环丙醇开环羰基化十步全合成 (±)-Phaeocaulisin A
我们报告了一种 (±)-phaeocaulisin A 的高效全合成方法,这是一种胍内酯倍半萜天然产物,具有复杂的四环骨架,内含一个氧杂内酯和一个融合的双环内酯、四个含氧立体中心和一个 8-氧杂双环[3.2.1]辛烷核心。我们的合成采用了新颖的钯催化环丙醇开环羰基化反应,以获得关键的γ-酮酯;化学和立体选择性醛醇环化反应,以形成七元碳环;以及级联酮化-内酯化反应,以构建所需的四环骨架。通过这些具有重要战略意义的 C-C 和 C-O 键形成转化,实现了 (±)-phaeocaulisin A 的十步全合成。我们进一步发展了环丙醇开环羰基化化学,为制备γ-酮酯提供了另一种方法。从生物学角度来看,具有 α-亚甲基 γ-丁内酯分子的倒数第二个中间体被鉴定为一种有前景的先导化合物,对三阴性或 HER2+ 乳腺癌细胞系具有抗癌增殖活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


