Cu-Catalyzed Asymmetric Three-Component Radical Acylarylation of Vinylarenes with Aldehydes and Aryl Boronic Acids
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The direct use of readily available aldehydes as acyl radical precursors has facilitated diverse three-component acylative difunctionalization reactions of alkenes, offering a powerful route to synthesize β-branched ketones. However, asymmetric three-component acylative difunctionalization of alkenes with aldehydes still remains elusive. Here we report a copper-catalyzed asymmetric three-component radical acylarylation of vinylarenes with aldehydes and aryl boronic acids. This method begins with acyl radical formation from an aldehyde via hydrogen atom transfer. The acyl radical adds to the alkene, forming a new benzylic radical that then undergoes copper-catalyzed enantioselective arylation. A chiral binaphthyl-tethered bisoxazoline ligand is essential for achieving high stereocontrol. This strategy enables the direct synthesis of a range of synthetically valuable chiral β,β-diaryl ketones from aldehydes and vinylarenes.
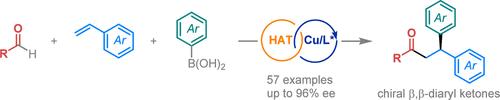
铜催化乙烯基醚与醛和芳基硼酸的不对称三组份自由基酰化反应
直接使用现成的醛作为酰基前体,促进了烯烃的多种三组分酰基双官能化反应,为合成β-支链酮提供了一条强有力的途径。然而,烯烃与醛的不对称三组分酰基双官能化反应仍然难以实现。在此,我们报告了铜催化的乙烯基烯烃与醛和芳基硼酸的不对称三组分自由基酰基化反应。这种方法首先通过氢原子转移从醛中形成酰基。酰基与烯烃相加,形成新的苄基,然后在铜催化下进行对映选择性芳基化反应。手性二萘系双噁唑啉配体是实现高度立体控制的关键。通过这种策略,可以从醛和乙烯基烯烃中直接合成一系列具有合成价值的手性 β、β-二芳基酮。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


