Ferroptosis to Pyroptosis Regulation by Iron-Based Nanocatalysts for Enhanced Tumor Immunotherapy
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Immunogenic cell death serves as a pivotal mechanism in enhancing antitumor immunotherapy by engaging both innate and adaptive immune responses. However, a key unanswered question is which mode of cell death, particularly ferroptosis or pyroptosis, serves as the optimal pathway for activating the immune response. In this study, we introduce an innovative iron-based nanocatalytic medicine that strategically regulates ferroptosis to pyroptosis to augment antitumor immunotherapy. By harnessing the intricate interplay between iron and carbonyl cyanide m-chlorophenyl hydrazone (CP), we engineered the nanomedicine which is capable of regulating ferroptosis to the more immunogenic pyroptosis within tumor cells. In vitro analyses revealed that the treatment with CP-encapsulated iron-based nanomedicine (HFCP) can effectively induce pyroptosis of cancer cells, exhibiting greatly enhanced efficacy in eradicating tumor cells and stimulating immune responses compared to the ferroptosis-inducing counterpart without CP incorporation (iron alone). Resultantly, HFCP not only effectively inhibited primary tumor growth but also suppressed the growth of untreated distant tumors to a large extent, underscoring a notably induced immune memory. Taken together, these results indicate that HFCP-induced pyroptosis offers a significantly more powerful approach to tumor immunotherapy than ferroptosis, offering promising potentials for achieving long-term immunotherapeutic outcomes through the reversal of the immunosuppressive tumor microenvironment and the effective regulation of immunogenic cell death modes.
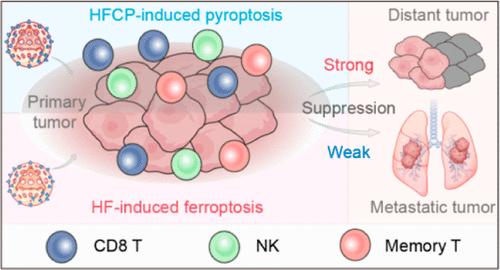
铁基纳米催化剂的铁跃迁到跃迁调控功能可增强肿瘤免疫疗法
免疫性细胞死亡是通过调动先天性和适应性免疫反应增强抗肿瘤免疫疗法的关键机制。然而,一个关键的未决问题是,哪种细胞死亡模式,尤其是铁凋亡还是热凋亡,是激活免疫反应的最佳途径。在这项研究中,我们介绍了一种创新的铁基纳米催化药物,它能策略性地调节铁凋亡和热凋亡,从而增强抗肿瘤免疫疗法。通过利用铁和羰基氰化间氯苯腙(CP)之间错综复杂的相互作用,我们设计出了一种纳米药物,它能够将肿瘤细胞内的铁突变调节为更具有免疫原性的热突变。体外分析表明,CP包封的铁基纳米药物(HFCP)能有效诱导癌细胞发生热休克,与未包封CP的诱导铁休克的纳米药物(单独使用铁)相比,HFCP在消灭肿瘤细胞和刺激免疫反应方面的功效大大增强。因此,HFCP 不仅能有效抑制原发性肿瘤的生长,还能在很大程度上抑制未经治疗的远处肿瘤的生长,从而显著诱导免疫记忆。综上所述,这些结果表明,HFCP 诱导的热蛋白沉积为肿瘤免疫治疗提供了一种比铁蛋白沉积更强大的方法,通过逆转免疫抑制性肿瘤微环境和有效调节免疫原性细胞死亡模式,为实现长期免疫治疗效果提供了广阔的前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


