Photophysics of 3-Carboxy-Salicylideneaniline in Rare Earth Metal Complexes with Silicon-Containing Schiff Base Ligands
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The investigations reported in this work concern the capabilities of the 3-carboxy-salicylidene unit in three dicompartmental Schiff bases with a flexible spacer bearing silane or siloxane units to efficiently coordinate rare earth and transition-metal ions as well as the efficiency to act as antenna or organometallic antenna for Ln(III) sensitization. The Ln(III) complexation studies of the H4L1, H4L2, and H4L3 ligands were performed by spectrophotometric titration. The selectivity toward seven Ln(III) ions and the trend of the stability constants are analyzed in connection with the structure of the ligands. In order to find the energetic factors that impact the efficiency of Ln(III) sensitization, the corresponding Gd(III) complexes were prepared and were in-depth analyzed from the point of view of the photophysical properties. The investigations were carried out using time-resolved emission and transient absorption spectroscopy. Phosphorescence data prove the unhindered path of the ligands to the triplet state and the probability to transfer the excitation energy to the Ln(III) ion. Applicability of two selected Gd(III) complexes in detection and recognition of transition and heavy metal ions has been studied with a double purpose: to evaluate their sensitivity and recognition ability so that they could be applied in pollutants detection and to estimate the capability of the M(II)-Schiff base unit as an organometallic antenna for Visible emission sensitization. The Gd(III) complex with a long π-conjugation showed multiple fluorescence responses to various kinds of 3d ions, which gave it pattern recognition ability. The discrimination power was finally analyzed by a principal component analysis.
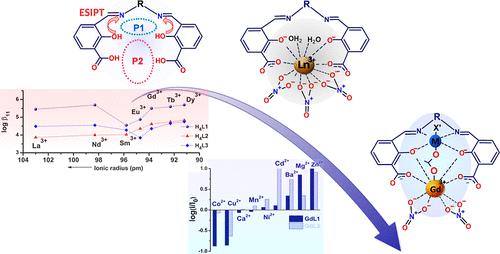
稀土金属络合物中的 3-羧基-水杨酰苯胺与含硅希夫碱配体的光物理关系
本研究报告所涉及的研究内容是三个二室席夫碱中的 3-羧基-水杨基单元与带有硅烷或硅氧烷单元的柔性间隔物有效配位稀土和过渡金属离子的能力,以及作为天线或有机金属天线对 Ln(III)敏化的效率。通过分光光度滴定法对 H4L1、H4L2 和 H4L3 配体进行了 Ln(III) 络合研究。结合配体的结构分析了对七种 Ln(III)离子的选择性和稳定性常数的变化趋势。为了找到影响 Ln(III)敏化效率的能量因素,制备了相应的 Gd(III)配合物,并从光物理特性的角度进行了深入分析。研究采用了时间分辨发射光谱和瞬态吸收光谱。磷光数据证明了配体通向三重态的路径畅通无阻,以及将激发能量转移到 Ln(III)离子上的可能性。我们研究了两种选定的 Gd(III) 复合物在检测和识别过渡金属和重金属离子方面的适用性,目的有二:评估它们的灵敏度和识别能力,以便将它们应用于污染物检测;以及评估 M(II)-Schiff 碱单元作为有机金属天线在可见光发射敏化方面的能力。具有长π共轭的钆(III)配合物对多种 3d 离子表现出多种荧光响应,这赋予了它模式识别能力。最后通过主成分分析对其识别能力进行了分析。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


