Depolymerization and Etching of Poly(lactic acid) via TiCl4 Vapor Phase Infiltration
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
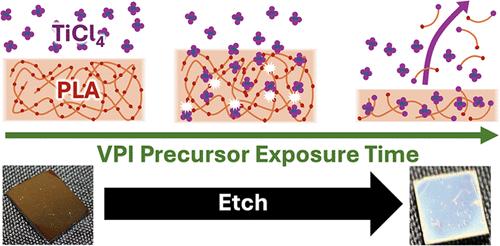
This study investigates the use of TiCl4 vapor phase infiltration (VPI) to cleave ester groups in the main chain of a polymer and drive depolymerization and film etching. Prior investigations have demonstrated that the infiltration of TiCl4 into PMMA results in dealkylation of its ester bond, cleaving its side groups. This study investigates the VPI of TiCl4 into poly(lactic acid), which is a prototypical polymer with an ester group in its main chain. Utilizing in situ quartz crystal microbalance (QCM) measurements and spectroscopic ellipsometry, PLA is observed to depolymerize readily at 135 °C with extended TiCl4 precursor exposure, resulting in significant thickness and mass reduction, whereas at 90 °C, depolymerization is significantly slower and etching is negligible. Utilizing Fourier transform infrared spectroscopy (FTIR), X-ray photoelectron spectroscopy (XPS), and a residual gas analyzer (RGA), dealkylation is shown to be the primary depolymerization mechanism. FTIR and XPS analyses reveal the consumption of carbonyl and methoxy groups and the emergence of hydroxyl, chlorine, and titanium moieties. In situ RGA measurements provide further insights into the byproducts formed during the TiCl4 and water exposure steps, indicating that the depolymerized components undergo further breakdown into other substances. Residuals left after 135 °C TiCl4 VPI are easily removed with a 0.1 M HCl aqueous solution. These findings highlight the expanding functionality of VPI, revealing its capability as both an additive and subtractive process and suggesting its broader applications.
通过 TiCl4 气相渗透实现聚(乳酸)的解聚和蚀刻
本研究探讨了如何利用 TiCl4 气相渗透(VPI)来裂解聚合物主链中的酯基,并推动解聚和薄膜蚀刻。先前的研究表明,将 TiCl4 渗入 PMMA 会导致其酯键脱烷基,从而裂解其侧基。本研究调查了 TiCl4 与聚乳酸的 VPI 关系,聚乳酸是一种主链中带有酯基的典型聚合物。利用原位石英晶体微天平(QCM)测量和光谱椭偏仪,观察到聚乳酸在 135 °C、TiCl4 前驱体暴露时间延长的情况下很容易发生解聚,导致厚度和质量显著降低,而在 90 °C时,解聚速度明显减慢,蚀刻可以忽略不计。利用傅立叶变换红外光谱(FTIR)、X 射线光电子能谱(XPS)和残余气体分析仪(RGA),脱烷基化被证明是主要的解聚机制。傅立叶变换红外光谱和 XPS 分析显示了羰基和甲氧基的消耗以及羟基、氯和钛分子的出现。原位 RGA 测量进一步揭示了在 TiCl4 和水暴露步骤中形成的副产品,表明解聚成分会进一步分解成其他物质。135 °C TiCl4 VPI 后的残留物很容易用 0.1 M HCl 水溶液去除。这些发现凸显了 VPI 不断扩展的功能,揭示了其作为添加剂和减量剂的能力,并暗示了其更广泛的应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


