The Influence of Solvent on Surface Adsorption and Desorption
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
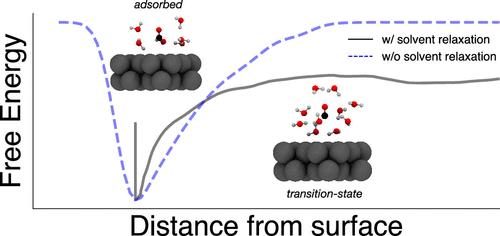
The adsorption and desorption of reactants and products from a solid surface are essential for achieving sustained surface chemical reactions. At a liquid–solid interface, these processes can involve the collective reorganization of interfacial solvent molecules in order to accommodate the adsorbing or desorbing species. Identifying the role of solvent in adsorption and desorption is important for advancing our understanding of surface chemical rates and mechanisms and enabling the rational design and optimization of surface chemical systems. In this manuscript, we use all-atom molecular dynamics simulation and transition path sampling to identify water’s role in the desorption of CO from a Pt(100) surface in contact with liquid water. We demonstrate that the solvation of CO, as quantified by the water coordination number, is an essential component of the desorption reaction coordinate. We use meta dynamics to compute the desorption free energy surface and conclude based on its features that desorption proceeds via a two-step mechanism whereby the final detachment of CO from the surface is preceded by the formation of a nascent solvation shell.
溶剂对表面吸附和解吸的影响
反应物和产物在固体表面的吸附和解吸对实现持续的表面化学反应至关重要。在液固界面上,这些过程可能涉及界面溶剂分子的集体重组,以适应吸附或解吸物种。确定溶剂在吸附和解吸过程中的作用对于加深我们对表面化学反应速率和机理的理解以及合理设计和优化表面化学体系非常重要。在本手稿中,我们利用全原子分子动力学模拟和过渡路径采样来确定水在 CO 从与液态水接触的 Pt(100)表面解吸过程中的作用。我们证明,以水配位数量化的 CO 溶解是解吸反应坐标的重要组成部分。我们利用元动力学计算了解吸自由能表面,并根据其特征得出结论:解吸是通过两步机制进行的,即在 CO 最终脱离表面之前,先形成一个新生的溶解壳。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


