Electrolyte Design via Cation–Anion Association Regulation for High-Rate and Dendrite-Free Zinc Metal Batteries at Low Temperature
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
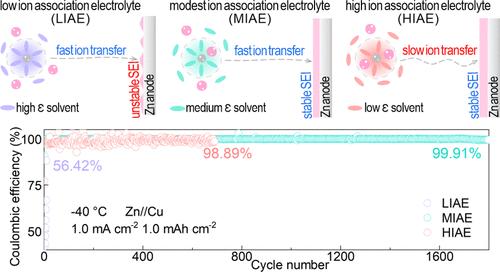
Low-temperature zinc metal batteries (ZMBs) are highly challenged by Zn dendrite growth, especially at high current density. Here, starting from the intermolecular insights, we report a cation–anion association modulation strategy by matching different dielectric constant solvents and unveil the relationship between cation–anion association strength and Zn plating/stripping performance at low temperatures. The combination of comprehensive characterizations and theoretical calculations indicates that moderate ion association electrolytes with high ionic conductivity (12.09 mS cm–1 at −40 °C) and a stable anion-derived solid electrolyte interphase (SEI) jointly account for highly reversible and dendrite-free Zn plating/stripping behavior, resulting in high-rate and ultrastable cycle performance at low temperature. As a result, at −40 °C, Zn//Zn cells can stably cycle over 400 h at 5 mA cm–2 and 10 mAh cm–2 and Zn//Cu cells exhibit an exceptional average Coulombic efficiency (CE) of 99.91% for 1800 cycles at 1 mA cm–2 and 1 mAh cm–2. Benefiting from enhanced low-temperature Zn plating/stripping performance, Zn//PANI full cells stably operate for 12,000 cycles at 0.5A g–1 and 35,000 cycles at 5 A g–1. Impressively, at −60 °C, Zn//Cu cells still display a high average CE of 99.68% for 2000 cycles. This work underscores the crucial effect of cation–anion association regulation for high-rate and dendrite-free Zn metal anodes, deepening the understanding of intermolecular interaction insights for low-temperature electrolyte design.
通过阳离子-阴离子关联调节设计电解质,实现低温下的高倍率和无枝晶型锌金属电池
低温锌金属电池(ZMB)受到锌枝晶生长的严峻挑战,尤其是在高电流密度下。在此,我们从分子间关系的角度出发,报告了一种通过匹配不同介电常数溶剂的阳离子-阴离子关联调制策略,并揭示了阳离子-阴离子关联强度与低温锌电镀/剥离性能之间的关系。综合表征和理论计算结果表明,具有高离子电导率(-40 °C时为12.09 mS cm-1)的适度离子关联电解质和稳定的阴离子衍生固体电解质相(SEI)共同促成了高度可逆和无树枝状突起的锌电镀/剥离行为,从而在低温条件下实现了高速率和超稳定的循环性能。因此,在-40 °C条件下,锌/锌电池在5 mA cm-2和10 mAh cm-2条件下可稳定循环400小时以上,而锌/铜电池在1 mA cm-2和1 mAh cm-2条件下循环1800次,平均库仑效率(CE)达到99.91%。得益于增强的低温镀锌/剥离性能,Zn//PANI 全电池在 0.5A g-1 电流条件下可稳定运行 12,000 次,在 5A g-1 电流条件下可稳定运行 35,000 次。令人印象深刻的是,在-60 °C条件下,Zn//Cu电池在2000次循环中仍然显示出99.68%的高平均CE值。这项研究强调了阳离子-阴离子关联调节对高倍率和无枝晶锌金属阳极的关键作用,加深了人们对分子间相互作用的理解,有助于低温电解液的设计。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


