Boron–pyridine nitrogen cooperative catalytic conversion of carbon dioxide and epoxides to cyclic carbonates†
IF 4.4
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
A type of N-heterocyclic carbene (NHC)-diboron adduct was used as a catalyst for the coupling of CO2 with epoxides. A multifunctional organocatalyst, which contains both boron as a Lewis acidic site and pyridine nitrogen as a Lewis basic site, offers an efficient alternative to traditional organoboron catalysts for the conversion of CO2 and epoxides into cyclic carbonates. The NHC-diboron adduct, in combination with tetrabutylammonium iodide (TBAI) as a nucleophile, catalyzes the coupling of CO2 and epoxides to obtain the cyclic carbonates in high yields under relatively mild conditions (50 °C and 1 MPa of CO2). The cooperative activation of NHC-diboron adducts was elaborated by in situ infrared spectroscopy and electrospray ionization-high-resolution mass spectrometry (ESI-HRMS). These results suggested that the Lewis acid boron center in NHC-diboron adduct activates the epoxide by coordination with its oxygen atom to promote ring opening and that the pyridine nitrogen acts as a Lewis basic site to activate CO2.
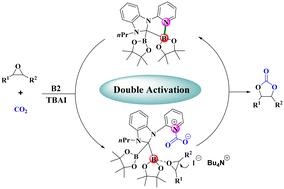
硼-吡啶氮协同催化二氧化碳和环氧化物转化为环状碳酸盐†。
一种 N-杂环碳烯(NHC)-二硼加合物被用作二氧化碳与环氧化物偶联的催化剂。这种多功能有机催化剂既含有作为路易斯酸性位点的硼,又含有作为路易斯碱性位点的吡啶氮,可有效替代传统的有机硼催化剂,将二氧化碳和环氧化物转化为环状碳酸盐。NHC 二硼加合物与作为亲核剂的碘化四丁基铵(TBAI)结合,在相对温和的条件下(50 °C 和 1 兆帕二氧化碳)催化了二氧化碳和环氧化物的偶联反应,从而获得了高产率的环碳酸盐。原位红外光谱和电喷雾离子化-高分辨质谱(ESI-HRMS)分析了 NHC-二硼加合物的协同活化。这些结果表明,NHC-二硼酸加合物中的路易斯酸硼中心通过与环氧化物的氧原子配位来激活环氧化物,从而促进开环,而吡啶氮则作为路易斯碱性位点激活二氧化碳。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


