Heterogeneous oxidative upcycling of polystyrene plastics to benzoic acid under air conditions†
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Plastics with inter-monomer C–C bond linkages comprise more than 70% of all plastics production, but their chemical recycling and upcycling usually require harsh conditions due to the inertness of C–C bonds, hindering their utilization efficiency. Here, we develop an oxidative upcycling strategy to convert polystyrene (PS) waste into benzoic acid over a NiO/TiO2 catalyst. This system is carried out in an environmentally friendly manner in an aqueous phase by using air as the oxidant, and up to 51.1% carbon yield of benzoic acid is obtained at 200 °C, 1 MPa air and 18 h. The conversion of real-life PS plastics is also successfully demonstrated. The reaction mechanism is further investigated by capturing radicals and intermediates during the oxidative reaction, confirming ·O2− radicals as the reactive oxygen species. A possible oxidative mechanism was proposed: the ·O2− radicals first activated the C–H bonds in the aliphatic portion of PS to generate carbonyl groups or CC bonds; then, through attack of the weak CC bonds, the polymer was constantly depolymerized to smaller oxygenated oligomers, dimers and finally the target product, benzoic acid. This work has provided a promising and green polystyrene upcycling strategy.
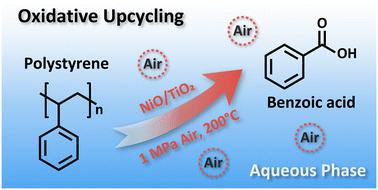
聚苯乙烯塑料在空气条件下的异相氧化上升循环生成苯甲酸†。
具有单体间 C-C 键连接的塑料占塑料总产量的 70% 以上,但由于 C-C 键的惰性,其化学回收和升级再循环通常需要苛刻的条件,从而阻碍了其利用效率。在此,我们开发了一种氧化上升循环策略,通过 NiO/TiO2 催化剂将聚苯乙烯(PS)废料转化为苯甲酸。该系统以空气为氧化剂,在水相环境中以环境友好的方式进行,在 200 ℃、1 兆帕空气和 18 小时的条件下,苯甲酸的碳收率高达 51.1%。通过捕捉氧化反应过程中的自由基和中间产物,进一步研究了反应机理,确认 -O2- 自由基为活性氧物种。提出了一种可能的氧化机制:-O2- 自由基首先激活 PS 脂肪族部分的 C-H 键,生成羰基或 CC 键;然后,通过攻击弱 CC 键,聚合物不断解聚成更小的含氧低聚物、二聚物,最后生成目标产物苯甲酸。这项工作提供了一种前景广阔的绿色聚苯乙烯升级再循环策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
文献相关原料
公司名称
产品信息
麦克林
Titanium dioxide (TiO2)
麦克林
Fe(NO3)3·9H2O
麦克林
polystyrene (PS)
麦克林
2,6-di-tert-butyl-4-methylphenol (BHT)
麦克林
tert-butanol
麦克林
1,4-benzoquinone
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


