Flow chemistry enhances catalytic alcohol-to-alkene dehydration†
IF 4.4
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Hf(OTf)4 was identified as an excellent catalyst for the low temperature (180 °C) dehydration of 1-hexanol to hexenes and 2-methyl-1-butanol to 2-methylbutenes. Batch conditions limited yields of alkene to 50% despite >90% conversions of 1-hexanol, 16% yield and 55% conversion for 2-methyl-1-butanol, but dramatically better yields were achieved using flow chemistry. For 2-methyl-1-butanol, steady-state conditions were achieved at 180 °C at flow rates of 0.1–0.2 mL min−1 that gave excellent mass balance and allowing selectivities and activities to be meaningfully compared. Hf(OTf)4 was the most active (51 h−1) with a selectivity of 50% at 50% conversion. Optimising for the production of purer alkene was achieved by raising the pressure producing 2.1 g h−1 of 2-methylbutenes (up to 98% pure by mass).
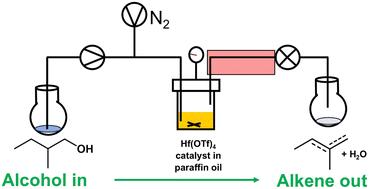
流动化学增强了催化醇-烯脱水能力†。
Hf(OTf)4 被确定为低温(180 °C)脱水将 1-己醇转化为己烯和将 2-甲基-1-丁醇转化为 2-甲基丁烯的极佳催化剂。尽管 1-己醇的转化率为 90%,2-甲基-1-丁醇的转化率为 55%,但批处理条件限制了烯的产量,仅为 50%。对于 2-甲基-1-丁醇,在 180 °C、流速为 0.1-0.2 mL min-1 的条件下实现了稳态,达到了极佳的质量平衡,从而可以对选择性和活性进行有意义的比较。Hf(OTf)4 的活性最高(51 h-1),在 50% 转化率下的选择性为 50%。通过提高压力,生产出 2.1 g h-1 的 2-甲基丁烯(纯度高达 98%),从而优化了更纯烯烃的生产。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


