Catalytic Concerted SNAr Reactions of Fluoroarenes by an Organic Superbase
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
We herein propose that the catalytic concerted SNAr reaction is a powerful method to prepare functionalized aromatic scaffolds. Classic stepwise SNAr reactions involving addition/elimination processes require the use of electron-deficient aromatic halides to stabilize Meisenheimer intermediates, despite their widespread use in medicinal chemistry research. Recent efforts have been made to develop concerted SNAr reactions involving a single transition state, allowing the use of electron-rich substrates based on the use of stoichiometric amounts of strong bases or reactive nucleophiles. This study demonstrates that, without the use of such reagents, the organic superbase t-Bu-P4 efficiently catalyzes the concerted SNAr reactions of aryl fluorides regardless of their electronic nature. The key to establishing this system is the dual activation of aryl fluoride and anionic nucleophiles by the t-Bu-P4 catalyst. Furthermore, this catalysis allows excellent functional group tolerance, utilization of diverse nucleophiles, and late-stage functionalization of bioactive compound derivatives. These findings make possible diverse applications in chemical synthesis and pharmaceutical development.
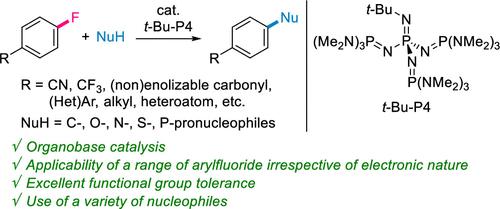
有机超级碱催化氟烯烃的 SNAr 协同反应
我们在此提出,催化协同 SNAr 反应是制备功能化芳香族支架的有力方法。涉及加成/消除过程的经典逐步 SNAr 反应需要使用缺电子的芳香卤化物来稳定迈森海默中间体,尽管它们在药物化学研究中得到了广泛应用。最近,人们努力开发涉及单一过渡态的协同 SNAr 反应,在使用等量强碱或活性亲核物的基础上,允许使用富电子底物。本研究表明,在不使用此类试剂的情况下,有机超级碱 t-Bu-P4 可以有效催化芳基氟化物的协同 SNAr 反应,而不管其电子性质如何。建立这一体系的关键在于 t-Bu-P4 催化剂对芳基氟化物和阴离子亲核物的双重活化。此外,这种催化方法还具有出色的官能团耐受性,可利用各种亲核物,并可对生物活性化合物衍生物进行后期官能化。这些发现为化学合成和药物开发领域的多种应用提供了可能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


