Determination of Carboxyl Dissociation Degree and pKa in Weak Polyelectrolyte Membranes via POT Titration and FTIR Analysis for Clean Technologies in Sustainability
IF 5.1
1区 化学
Q1 POLYMER SCIENCE
引用次数: 0
Abstract
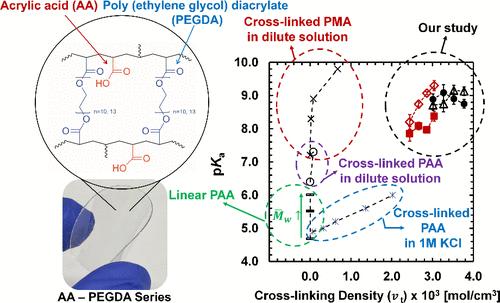
Charged polymer membranes play a crucial role in clean technologies for sustainability. Previously, we have designed a series of weak polyelectrolyte membranes, i.e., acrylic acid–poly(ethylene glycol) diacrylate (AA-PEGDA) networks with a wide ion-exchange capacity range (IEC: 0–4 mequiv/g) and limited water swelling. An acrylic acid (AA) monomer was used to control the amount of dissociated charged (COO–) groups versus pH. To systematically quantify the dissociated charged (COO–) group content in the polymers, we reported the degree of ionization (α) and negative logarithm of acid dissociation constant (pKa) of AA-PEGDA series at varied external pH via conventional potentiometric titration (POT titration) and widely available ATR-FTIR analysis. Contact angle measurement was also used to record the surface hydrophilicity vs pH to support the analysis. Overall, the degree of ionization (α) vs pH trends via three different methods well-align to each other, indicating that three methods which (1) measure pH changes in a solution phase (POT titration), (2) directly probe dissociated COO– groups in a polymer phase (ATR-FTIR) and (3) record surface hydrophilicity (contact angle) reasonably describe the molecular-level, physical picture of dissociation process in the polymers. For all compositions, as external pH increases (pH = 5–12), the degree of ionization (α) also increases between 0 and 1, following the modified Henderson–Hasselbalch equation. As the maximum ion-exchange capacity (mIEC) increases (AA content increases and PEGDA cross-linker content decreases), the decreased cross-linking density and simultaneously increased water content dominantly reduce the overall electrostatic repulsion, showing decreased pKa. Our system shows higher cross-linking density and was tested in dilute conditions, showing increased pKa values compared with those of other polymers in the literature. To the best of our knowledge, this is the first time the charged group concentration (mIEC) and cross-linking density have been systematically changed to record the pKa trend in thin film forms using a single polymer network system. Our AA-PEGDA series can provide a platform to advance our understanding of the dissociation process in weak polyelectrolyte polymers.
通过 POT 滴定和傅立叶变换红外分析法确定弱聚电解质膜中的羧基解离度和 pKa,促进清洁技术的可持续发展
带电聚合物膜在可持续发展的清洁技术中发挥着至关重要的作用。此前,我们设计了一系列弱聚电解质膜,即丙烯酸-聚(乙二醇)二丙烯酸酯(AA-PEGDA)网络,其离子交换容量范围很宽(IEC:0-4 mequiv/g),水膨胀有限。丙烯酸(AA)单体用于控制离解带电(COO-)基团的数量与 pH 值的关系。为了系统地量化聚合物中离解带电(COO-)基团的含量,我们通过传统的电位滴定法(POT 滴定法)和广泛使用的 ATR-FTIR 分析法,报告了 AA-PEGDA 系列在不同外部 pH 值下的电离度(α)和酸解离常数负对数(pKa)。此外,还使用接触角测量法记录表面亲水性与 pH 值的关系,为分析提供支持。总体而言,三种不同方法得出的电离度(α)与 pH 值的变化趋势相互吻合,这表明以下三种方法都能合理地描述聚合物解离过程的分子级物理现象:(1) 测量溶液相中的 pH 值变化(POT 滴定法);(2) 直接探测聚合物相中解离的 COO- 基团(ATR-FTIR);(3) 记录表面亲水性(接触角)。对于所有成分,随着外部 pH 值的增加(pH = 5-12),电离度 (α)也在 0 和 1 之间增加,遵循修正的 Henderson-Hasselbalch 方程。随着最大离子交换容量(mIEC)的增加(AA 含量的增加和 PEGDA 交联剂含量的减少),交联密度的降低和同时水含量的增加在很大程度上降低了整体静电斥力,从而显示出 pKa 值的降低。与文献中的其他聚合物相比,我们的体系显示出更高的交联密度,并在稀释条件下进行了测试,显示出更高的 pKa 值。据我们所知,这是首次通过系统地改变带电基团浓度(mIEC)和交联密度来记录使用单一聚合物网络体系的薄膜形式的 pKa 变化趋势。我们的 AA-PEGDA 系列可为我们提供一个平台,帮助我们加深对弱聚电解质聚合物解离过程的理解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Macromolecules
工程技术-高分子科学
CiteScore
9.30
自引率
16.40%
发文量
942
审稿时长
2 months
期刊介绍:
Macromolecules publishes original, fundamental, and impactful research on all aspects of polymer science. Topics of interest include synthesis (e.g., controlled polymerizations, polymerization catalysis, post polymerization modification, new monomer structures and polymer architectures, and polymerization mechanisms/kinetics analysis); phase behavior, thermodynamics, dynamic, and ordering/disordering phenomena (e.g., self-assembly, gelation, crystallization, solution/melt/solid-state characteristics); structure and properties (e.g., mechanical and rheological properties, surface/interfacial characteristics, electronic and transport properties); new state of the art characterization (e.g., spectroscopy, scattering, microscopy, rheology), simulation (e.g., Monte Carlo, molecular dynamics, multi-scale/coarse-grained modeling), and theoretical methods. Renewable/sustainable polymers, polymer networks, responsive polymers, electro-, magneto- and opto-active macromolecules, inorganic polymers, charge-transporting polymers (ion-containing, semiconducting, and conducting), nanostructured polymers, and polymer composites are also of interest. Typical papers published in Macromolecules showcase important and innovative concepts, experimental methods/observations, and theoretical/computational approaches that demonstrate a fundamental advance in the understanding of polymers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


