Iron-Mediated Segment Coupling of Alkenols with Acceptors via C–C Radical Translocation and Remote Oxidative 1,5/6-Hydrogen Atom Transfer
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Iron-mediated segment coupling followed by oxidative 1,5/6-hydrogen atom transfer (HAT) for synthesis of ε-oxo alkene derivatives is developed. This transformation involved translocation of the radical from H-to-C-to-C-to-C followed by the oxidation under MHAT conditions providing rapid access to 1,6/1,7-keto functionalized esters/ketone/sulfones/phosphonates/arenes. The different outcomes of coupling with acceptors could be explained by bond dissociation energies (BDEs), and mechanistic insights were gained through control experiments, including deuterium labeling studies.
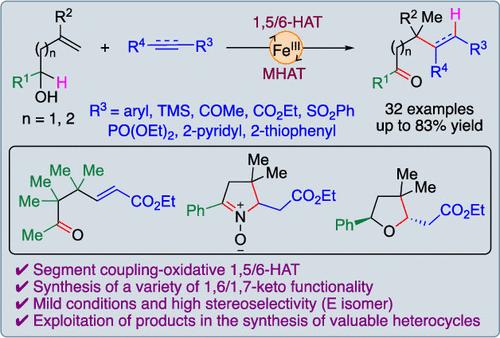
铁介导的烯醇与受体通过 C-C 自由基转移和 1,5/6-氢原子远程氧化转移的段耦合
该研究开发了铁介导的段偶联,然后是氧化性 1,5/6 氢原子转移 (HAT),用于合成ε-氧代烯衍生物。这种转化涉及自由基从 H 到 C 到 C 到 C 的转移,然后在 MHAT 条件下进行氧化,从而快速获得 1,6/1,7-酮官能化的酯/酮/砜/膦酸盐/烯。与受体耦合的不同结果可以用键解离能(BDE)来解释,并通过对照实验(包括氘标记研究)获得了机理方面的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


